Structure ( IF 4.4 ) Pub Date : 2020-09-22 , DOI: 10.1016/j.str.2020.09.002 Tufa E Assafa 1 , Sukhendu Nandi 1 , Dariusz Śmiłowicz 2 , Laura Galazzo 1 , Markus Teucher 1 , Christina Elsner 1 , Stefanie Pütz 1 , Stephanie Bleicken 1 , Adeline Y Robin 3 , Dana Westphal 4 , Isabel Uson 5 , Raphael Stoll 1 , Peter E Czabotar 3 , Nils Metzler-Nolte 2 , Enrica Bordignon 1
|
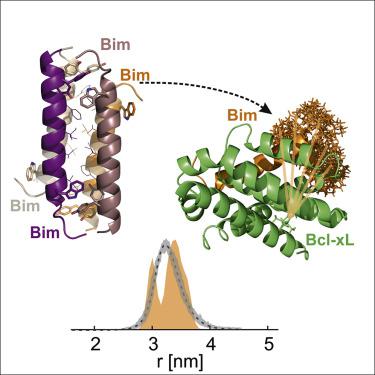
Bcl-2 proteins orchestrate the mitochondrial pathway of apoptosis, pivotal for cell death. Yet, the structural details of the conformational changes of pro- and antiapoptotic proteins and their interactions remain unclear. Pulse dipolar spectroscopy (double electron-electron resonance [DEER], also known as PELDOR) in combination with spin-labeled apoptotic Bcl-2 proteins unveils conformational changes and interactions of each protein player via detection of intra- and inter-protein distances. Here, we present the synthesis and characterization of pro-apoptotic BimBH3 peptides of different lengths carrying cysteines for labeling with nitroxide or gadolinium spin probes. We show by DEER that the length of the peptides modulates their homo-interactions in the absence of other Bcl-2 proteins and solve by X-ray crystallography the structure of a BimBH3 tetramer, revealing the molecular details of the inter-peptide interactions. Finally, we prove that using orthogonal labels and three-channel DEER we can disentangle the Bim-Bim, Bcl-xL-Bcl-xL, and Bim-Bcl-xL interactions in a simplified interactome.
中文翻译:

促凋亡 BimBH3 肽的生物物理表征揭示了一种意想不到的自缔合能力
Bcl-2 蛋白协调细胞凋亡的线粒体途径,对细胞死亡至关重要。然而,促凋亡和抗凋亡蛋白的构象变化及其相互作用的结构细节仍不清楚。脉冲偶极光谱(双电子-电子共振 [DEER],也称为 PELDOR)与自旋标记的凋亡 Bcl-2 蛋白相结合,通过检测蛋白质内和蛋白质间的距离来揭示每个蛋白质参与者的构象变化和相互作用。在这里,我们展示了携带半胱氨酸的不同长度的促凋亡 BimBH3 肽的合成和表征,用于用氮氧化物或钆自旋探针进行标记。我们通过 DEER 显示肽的长度在没有其他 Bcl-2 蛋白的情况下调节它们的同源相互作用,并通过 X 射线晶体学解析 BimBH3 四聚体的结构,揭示肽间相互作用的分子细节。最后,我们证明使用正交标签和三通道 DEER,我们可以在简化的交互组中解开 Bim-Bim、Bcl-xL-Bcl-xL 和 Bim-Bcl-xL 相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号