当前位置:
X-MOL 学术
›
Ecol. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ remediation of Cd(II) contaminated paddy fields with activated Ca Si mineral material derived from Potash feldspar and its mechanism
Ecological Engineering ( IF 3.9 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.ecoleng.2020.106052 Meiqing Zeng , Xiaohui Zhou , Jing Guo , Kaiyu Liu , Chubin Zhong , Yaochi Liu
Ecological Engineering ( IF 3.9 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.ecoleng.2020.106052 Meiqing Zeng , Xiaohui Zhou , Jing Guo , Kaiyu Liu , Chubin Zhong , Yaochi Liu
|
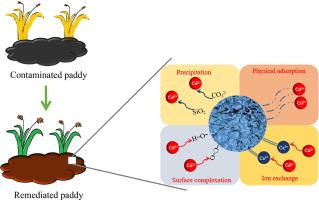
Abstract Continuous rice cultivation and excessive use of chemical fertilizers result in soil acidification and heavy metal pollution in southern China. An activated Ca Si mineral remediation material (CSRM) was innovatively prepared by two-stage modification (calcination with mixed salts and the hydrothermal activation) of primary aluminosilicate Potash feldspar. Based on the excellent results of previous pot experiments, this material was used for in situ remediation of cadmium contaminated paddy fields. Adding 4500 kg/hm2 of the material, the cadmium contents in rice and the available cadmium contents in soil was reduced significantly by 41.43%–73.32% and 16.67%–31.13%, accompanied by the increase of soil pH 0.68–0.82 and rice yield 4.24%–11.22%. To further study the remediation mechanism of cadmium, the composite was used for Cd(II) removal in aqueous solution, in combination with SEM, EDX, XPS, XRD, XRF, and BET analysis. The adsorption isothermal data was more suitable with the Langmuir model, and the kinetics can be well described by the pseudo-second-order model. The isothermal study also demonstrated an adsorption process on homogeneous surface along with monolayer adsorption and the maximum capacity obtained from the Langmuir model was 273.2 mg/g at 328 K. By instrumental analysis, it could be known that stable carbonates and silicates were formed by the interaction of materials with cadmium ions, and this process was feasible, spontaneous and endothermic. Ion exchange was found to be the most important chemical process, followed by precipitation, surface complexation and adsorption. The porosity of clay was another significant reason for the removal of heavy metals by adsorption mechanism.
更新日期:2020-12-01











































 京公网安备 11010802027423号
京公网安备 11010802027423号