Applied Clay Science ( IF 5.3 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.clay.2020.105844 Javiera Cervini-Silva , Gerardo Ruíz , José Manuel Hernández , Eduardo Palacios , Jorge Balmaseda , Maripaz Orta , Sergio Hernández
|
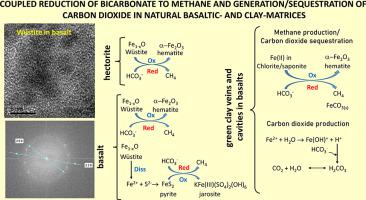
In nature, methane (CH4) is produced via both biotic and abiotic processes, yet little is known on the abiotic mechanism(s) on the hydrogenation of abiotic carbon (C) to produce CH4 at low temperatures. This study reports on the abiotic generation of CH4via Fischer-Tropsch type reactions at room temperature. Natural sources of mineral Fe2+ were reacted with aqueous bicarbonate under sterile and non-sterile conditions. The selected sources of mineral Fe2+ were well-characterized Ediacaran basalts and green clay veins in basalts from the Volyn-Brest continental flood basalt province and hectorite SHCa-1 from Hector, California. Quantitative X-ray powder diffraction results and the Mössbauer characteristics of these materials from the literature were supplemented with specific surface area values determined by N2 physisorption, high-resolution transmission electron microscopy and nanodiffraction analysis. The headspace concentration of the gases in the dispersions was related to the presence of bicarbonate and the mineral composition. Evidence presented herein confirmed that wüstite occurs naturally in SHCa-1, which is unprecedented. Of all mineral dispersions those containing wüstite (basalt MIB and SHCa-1) showed the highest concentration of CH4 and CO2, ranging from 1457 to 6329 μmol CH4 g solid−1 and from 2100 to 8771 μmol CO2 g solid−1, respectively. The production of CO2 was also registered in dispersions containing the sources of mineral Fe(II) chlorite and saponite, which ranged from 375 to 8180 μmol CO2 g solid−1 and from 480 to 1230 μmol CO2 g solid−1, respectively. Registered mineral transformations i) wüstite → hematite and ii) chlorite or saponite → hematite, and iii) wüstite → pyrite → jarosite were explained because bicarbonate and wüstite (chlorite or saponite) reduction and oxidation to CH4 and hematite; and wustite dissolution releasing Fe2+ that reacted with sulfide ions.
中文翻译:

碳酸氢盐的联接减少到甲烷和产生/在天然basaltic-和粘土矩阵二氧化碳封存在25℃≤ Ť ≤100℃
在自然界中,甲烷(CH 4)产生通过生物和非生物过程,但很少有人对非生物性碳(C)的氢化反应来制备CH非生物机制(多个)已知的4在低温下。这项研究报告了在室温下通过费-托型反应产生的CH 4非生物产生。天然的Fe 2+矿物源在无菌和非无菌条件下与碳酸氢盐水溶液反应。选定的Fe 2+矿物来源分别是来自Volyn-Brest大陆洪水玄武岩省的玄武岩中特征明确的Ediacaran玄武岩和绿色粘土脉,以及来自加利福尼亚州赫克托的锂蒙脱石SHCa-1。定量的X射线粉末衍射结果和这些材料从文献中获得的Mössbauer特性还通过N 2物理吸附,高分辨率透射电子显微镜和纳米衍射分析确定了比表面积值。分散液中气体的顶空浓度与碳酸氢盐和矿物成分的存在有关。本文提供的证据证实,白钨矿自然存在于SHCa-1中,这是前所未有的。在所有矿物分散体中,含辉石(玄武岩MIB和SHCa-1)的CH 4浓度最高。CH 2和CO 2分别为1457至6329μmolCH 4 g固体-1和2100至8771μmolCO 2 g固体-1。CO的产生2也被登记在含矿物的Fe(II)亚氯酸盐和皂石,其范围从375到8180的源极分散体微摩尔CO 2克固体-1和480〜1230微摩尔CO 2的固体克-1,分别。解释了注册的矿物转化i)钨铁矿→赤铁矿和ii)亚氯酸盐或皂石→赤铁矿,以及iii)钨铁矿→黄铁矿→黄钾铁矾,因为碳酸氢盐和锰铁矿(绿泥石或皂石)被还原并氧化成CH 4和赤铁矿;钙钛矿溶解后释放出与硫化物离子反应的Fe 2+。











































 京公网安备 11010802027423号
京公网安备 11010802027423号