当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical synthesis of a haemathrin sulfoprotein library reveals enhanced thrombin inhibition following tyrosine sulfation
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-09-21 , DOI: 10.1039/d0cb00146e Daniel Clayton 1, 2, 3, 4 , Sameer S. Kulkarni 1, 2, 3, 4 , Jessica Sayers 1, 2, 3, 4 , Luke J. Dowman 1, 2, 3, 4 , Jorge Ripoll-Rozada 5, 6, 7, 8 , Pedro José Barbosa Pereira 5, 6, 7, 8 , Richard J. Payne 1, 2, 3, 4, 9
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-09-21 , DOI: 10.1039/d0cb00146e Daniel Clayton 1, 2, 3, 4 , Sameer S. Kulkarni 1, 2, 3, 4 , Jessica Sayers 1, 2, 3, 4 , Luke J. Dowman 1, 2, 3, 4 , Jorge Ripoll-Rozada 5, 6, 7, 8 , Pedro José Barbosa Pereira 5, 6, 7, 8 , Richard J. Payne 1, 2, 3, 4, 9
Affiliation
|
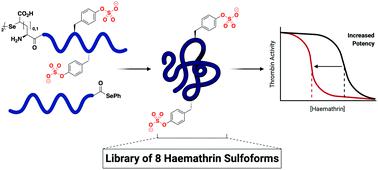
The haemathrins are tick-derived thrombin-inhibiting proteins predicted to be post-translationally sulfated. This study reports the ligation-based assembly of eight homogeneously sulfated variants of haemathrin-1 and haemathrin-2. Functional assays revealed a two orders-of-magnitude enhancement in thrombin-inhibitory potency by tyrosine sulfation, thus reinforcing the crucial role of this post-translational modification for the activity of anticoagulant proteins.
中文翻译:

血红蛋白磺蛋白文库的化学合成显示酪氨酸硫酸化后增强了对凝血酶的抑制作用
血红素是tick的凝血酶抑制蛋白,预计在翻译后会被硫酸化。这项研究报告了基于连接的组装的8个均被硫酸血红素1和血红素2变异体组装。功能测定显示酪氨酸硫酸化使凝血酶抑制能力增强了两个数量级,从而增强了这种翻译后修饰对抗凝蛋白活性的关键作用。
更新日期:2020-11-03
中文翻译:

血红蛋白磺蛋白文库的化学合成显示酪氨酸硫酸化后增强了对凝血酶的抑制作用
血红素是tick的凝血酶抑制蛋白,预计在翻译后会被硫酸化。这项研究报告了基于连接的组装的8个均被硫酸血红素1和血红素2变异体组装。功能测定显示酪氨酸硫酸化使凝血酶抑制能力增强了两个数量级,从而增强了这种翻译后修饰对抗凝蛋白活性的关键作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号