Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fattening chips: hypertrophy, feeding, and fasting of human white adipocytes in vitro
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-09-21 , DOI: 10.1039/d0lc00508h Benjamin D Pope 1 , Curtis R Warren 2 , Madeleine O Dahl 3 , Christina V Pizza 3 , Douglas E Henze 3 , Nina R Sinatra 3 , Grant M Gonzalez 3 , Huibin Chang 3 , Qihan Liu 3 , Aaron L Glieberman 3 , John P Ferrier 3 , Chad A Cowan 4 , Kevin Kit Parker 5
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-09-21 , DOI: 10.1039/d0lc00508h Benjamin D Pope 1 , Curtis R Warren 2 , Madeleine O Dahl 3 , Christina V Pizza 3 , Douglas E Henze 3 , Nina R Sinatra 3 , Grant M Gonzalez 3 , Huibin Chang 3 , Qihan Liu 3 , Aaron L Glieberman 3 , John P Ferrier 3 , Chad A Cowan 4 , Kevin Kit Parker 5
Affiliation
|
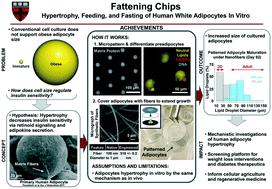
Adipose is a distributed organ that performs vital endocrine and energy homeostatic functions. Hypertrophy of white adipocytes is a primary mode of both adaptive and maladaptive weight gain in animals and predicts metabolic syndrome independent of obesity. Due to the failure of conventional culture to recapitulate adipocyte hypertrophy, technology for production of adult-size adipocytes would enable applications such as in vitro testing of weight loss therapeutics. To model adaptive adipocyte hypertrophy in vitro, we designed and built fat-on-a-chip using fiber networks inspired by extracellular matrix in adipose tissue. Fiber networks extended the lifespan of differentiated adipocytes, enabling growth to adult sizes. By micropatterning preadipocytes in a native cytoarchitecture and by adjusting cell-to-cell spacing, rates of hypertrophy were controlled independent of culture time or differentiation efficiency. In vitro hypertrophy followed a nonlinear, nonexponential growth model similar to human development and elicited transcriptomic changes that increased overall similarity with primary tissue. Cells on the chip responded to simulated meals and starvation, which potentiated some adipocyte endocrine and metabolic functions. To test the utility of the platform for therapeutic development, transcriptional network analysis was performed, and retinoic acid receptors were identified as candidate drug targets. Regulation by retinoid signaling was suggested further by pharmacological modulation, where activation accelerated and inhibition slowed hypertrophy. Altogether, this work presents technology for mature adipocyte engineering, addresses the regulation of cell growth, and informs broader applications for synthetic adipose in pharmaceutical development, regenerative medicine, and cellular agriculture.
中文翻译:

增肥芯片:人白色脂肪细胞体外肥大、喂养和禁食
脂肪是一种分布式器官,执行重要的内分泌和能量稳态功能。白色脂肪细胞肥大是动物适应性和适应不良体重增加的主要模式,可预测与肥胖无关的代谢综合征。由于传统培养无法重现脂肪细胞肥大,成人大小脂肪细胞的生产技术将使减肥疗法的体外测试等应用成为可能。为了在体外模拟适应性脂肪细胞肥大,我们受脂肪组织细胞外基质的启发,使用纤维网络设计并构建了脂肪芯片。纤维网络延长了分化脂肪细胞的寿命,使其能够生长到成人大小。通过在天然细胞结构中对前脂肪细胞进行微图案化并通过调整细胞间间距,可以独立于培养时间或分化效率来控制肥大率。体外肥大遵循类似于人类发育的非线性、非指数生长模型,并引起转录组变化,从而增加了与原代组织的整体相似性。芯片上的细胞对模拟的膳食和饥饿做出反应,从而增强了一些脂肪细胞的内分泌和代谢功能。为了测试该平台在治疗开发方面的实用性,进行了转录网络分析,并将视黄酸受体确定为候选药物靶点。药理调节进一步表明类维生素A信号传导的调节,其中激活加速,抑制减缓肥大。总而言之,这项工作提出了成熟脂肪细胞工程技术,解决了细胞生长的调节问题,并为合成脂肪在药物开发、再生医学和细胞农业中的更广泛应用提供了信息。
更新日期:2020-11-03
中文翻译:

增肥芯片:人白色脂肪细胞体外肥大、喂养和禁食
脂肪是一种分布式器官,执行重要的内分泌和能量稳态功能。白色脂肪细胞肥大是动物适应性和适应不良体重增加的主要模式,可预测与肥胖无关的代谢综合征。由于传统培养无法重现脂肪细胞肥大,成人大小脂肪细胞的生产技术将使减肥疗法的体外测试等应用成为可能。为了在体外模拟适应性脂肪细胞肥大,我们受脂肪组织细胞外基质的启发,使用纤维网络设计并构建了脂肪芯片。纤维网络延长了分化脂肪细胞的寿命,使其能够生长到成人大小。通过在天然细胞结构中对前脂肪细胞进行微图案化并通过调整细胞间间距,可以独立于培养时间或分化效率来控制肥大率。体外肥大遵循类似于人类发育的非线性、非指数生长模型,并引起转录组变化,从而增加了与原代组织的整体相似性。芯片上的细胞对模拟的膳食和饥饿做出反应,从而增强了一些脂肪细胞的内分泌和代谢功能。为了测试该平台在治疗开发方面的实用性,进行了转录网络分析,并将视黄酸受体确定为候选药物靶点。药理调节进一步表明类维生素A信号传导的调节,其中激活加速,抑制减缓肥大。总而言之,这项工作提出了成熟脂肪细胞工程技术,解决了细胞生长的调节问题,并为合成脂肪在药物开发、再生医学和细胞农业中的更广泛应用提供了信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号