当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition and disaggregation of amyloid β protein fibrils through conjugated polymer–core thermoresponsive micelles
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2020-09-21 , DOI: 10.1039/d0tb01863e Hao Geng 1, 2, 3, 4 , Hongbo Yuan 2, 3, 5, 6, 7 , Liang Qiu 2, 3, 5, 6, 7 , Dong Gao 2, 3, 5, 6, 7 , Yongqiang Cheng 8, 9, 10, 11, 12 , Chengfen Xing 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2020-09-21 , DOI: 10.1039/d0tb01863e Hao Geng 1, 2, 3, 4 , Hongbo Yuan 2, 3, 5, 6, 7 , Liang Qiu 2, 3, 5, 6, 7 , Dong Gao 2, 3, 5, 6, 7 , Yongqiang Cheng 8, 9, 10, 11, 12 , Chengfen Xing 1, 2, 3, 4, 5
Affiliation
|
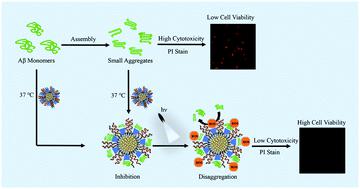
Excess aggregation of amyloid β peptide (Aβ) is a fatal cause of Alzheimer's disease (AD), which leads to physiological toxicity. Inhibiting and disaggregating the Aβ aggregates is an effective strategy to reduce physiological toxicity in neuronal cells. Herein, conjugated polymer-based thermoresponsive micelles (CPMs) were designed with an efficient thermoresponsive surface and a reactive-oxygen-species (ROS)-generating core. In this work, the CPMs exhibited a strong capability to capture the toxic Aβ aggregates at physiological temperature. Under white-light irradiation, ROS was generated in the CPMs, and the toxic Aβ aggregates were efficiently disaggregated through the oxidation of ROS, leading to appropriate Aβ homeostasis between aggregation and disaggregation and reduced the Aβ-induced cytotoxicity. Therefore, the multifunctional micelles of CPMs with both capturing shells and ROS functional cores present a promising strategy to reduce Aβ fibrillation-induced cytotoxicity.
中文翻译:

淀粉样β蛋白原纤维通过共轭聚合物核热响应性胶束的抑制和分解
淀粉样蛋白β肽(Aβ)的过度聚集是阿尔茨海默氏病(AD)的致命原因,导致生理毒性。抑制和分解Aβ聚集体是减少神经元细胞生理毒性的有效策略。本文中,基于共轭聚合物的热响应胶束(CPM)设计为具有有效的热响应表面和产生活性氧物种(ROS)的核心。在这项工作中,CPM在生理温度下表现出强大的捕获有毒Aβ聚集体的能力。在白光照射下,CPM中产生了ROS,并且通过ROS的氧化有效地分解了有毒的Aβ聚集体,从而导致了Aβ在聚集与分解之间的体内稳态,并降低了Aβ诱导的细胞毒性。因此,
更新日期:2020-11-03
中文翻译:

淀粉样β蛋白原纤维通过共轭聚合物核热响应性胶束的抑制和分解
淀粉样蛋白β肽(Aβ)的过度聚集是阿尔茨海默氏病(AD)的致命原因,导致生理毒性。抑制和分解Aβ聚集体是减少神经元细胞生理毒性的有效策略。本文中,基于共轭聚合物的热响应胶束(CPM)设计为具有有效的热响应表面和产生活性氧物种(ROS)的核心。在这项工作中,CPM在生理温度下表现出强大的捕获有毒Aβ聚集体的能力。在白光照射下,CPM中产生了ROS,并且通过ROS的氧化有效地分解了有毒的Aβ聚集体,从而导致了Aβ在聚集与分解之间的体内稳态,并降低了Aβ诱导的细胞毒性。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号