当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioinspired Nickel Complexes Supported by an Iron Metalloligand.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-20 , DOI: 10.1021/acs.inorgchem.0c02041 Jacob R Prat , Carlo A Gaggioli , Ryan C Cammarota , Eckhard Bill 1 , Laura Gagliardi , Connie C Lu
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-09-20 , DOI: 10.1021/acs.inorgchem.0c02041 Jacob R Prat , Carlo A Gaggioli , Ryan C Cammarota , Eckhard Bill 1 , Laura Gagliardi , Connie C Lu
Affiliation
|
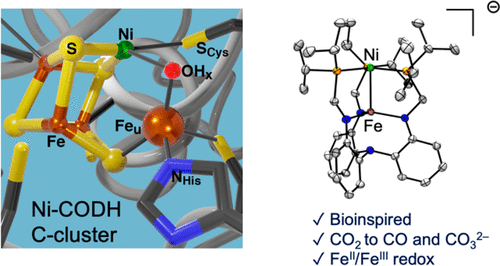
Nature utilizes multimetallic sites in metalloenzymes to enable multielectron chemical transformations at ambient conditions and low overpotentials. One such example of multimetallic cooperativity can be found in the C-cluster of Ni–carbon monoxide dehydrogenase (CODH), which interconverts CO and CO2. Toward a potential functional model of the C-cluster, a family of Ni–Fe bimetallic complexes was synthesized that contain direct metal–metal bonding interactions. The complexes were characterized by X-ray crystallography, various spectroscopies (NMR, EPR, UV−vis, Mössbauer), and theoretical calculations. The Ni–Fe bimetallic system has a reversible Fe(III)/Fe(II) redox couple at −2.10 V (vs Fc+/Fc). The Fe-based “redox switch” can turn on CO2 reactivity at the Ni(0) center by leveraging the Ni→Fe dative interaction to attenuate the Ni(0) electron density. The reduced Ni(0)Fe(II) species mediated the formal two-electron reduction of CO2 to CO, providing a Ni–CO adduct and CO32– as products. During the reaction, an intermediate was observed that is proposed to be a Ni–CO2 species.
中文翻译:

铁金属配体支持的生物启发镍配合物。
大自然利用金属酶中的多金属位点在环境条件和低超电势下实现多电子化学转化。可以在将CO和CO 2相互转化的Ni-一氧化碳脱氢酶(CODH)的C簇中找到这种多金属协同性的例子。为了建立潜在的C簇功能模型,我们合成了一个Ni-Fe双金属配合物族,其中包含直接的金属-金属键相互作用。通过X射线晶体学,各种光谱学(NMR,EPR,UV-vis,Mössbauer)和理论计算来表征配合物。Ni-Fe双金属系统在-2.10 V(vs Fc + / Fc)下具有可逆的Fe(III)/ Fe(II)氧化还原对。铁基“氧化还原开关”可以接通CO 2通过利用Ni→Fe介导的相互作用来减弱Ni(0)电子密度,从而在Ni(0)中心具有较高的反应活性。还原的Ni(0)Fe(II)物种介导了CO 2正式转化为CO的两电子,从而提供了Ni-CO加合物和CO 3 2–作为产物。在反应过程中,观察到一种中间体,该中间体被认为是Ni–CO 2物种。
更新日期:2020-10-05
中文翻译:

铁金属配体支持的生物启发镍配合物。
大自然利用金属酶中的多金属位点在环境条件和低超电势下实现多电子化学转化。可以在将CO和CO 2相互转化的Ni-一氧化碳脱氢酶(CODH)的C簇中找到这种多金属协同性的例子。为了建立潜在的C簇功能模型,我们合成了一个Ni-Fe双金属配合物族,其中包含直接的金属-金属键相互作用。通过X射线晶体学,各种光谱学(NMR,EPR,UV-vis,Mössbauer)和理论计算来表征配合物。Ni-Fe双金属系统在-2.10 V(vs Fc + / Fc)下具有可逆的Fe(III)/ Fe(II)氧化还原对。铁基“氧化还原开关”可以接通CO 2通过利用Ni→Fe介导的相互作用来减弱Ni(0)电子密度,从而在Ni(0)中心具有较高的反应活性。还原的Ni(0)Fe(II)物种介导了CO 2正式转化为CO的两电子,从而提供了Ni-CO加合物和CO 3 2–作为产物。在反应过程中,观察到一种中间体,该中间体被认为是Ni–CO 2物种。











































 京公网安备 11010802027423号
京公网安备 11010802027423号