当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low- to Room-Temperature Dehydrogenation of Dimethylamine Borane Facilitated by Ionic Liquids: Molecular Modeling and Experimental Studies
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.energyfuels.0c01896 Debashis Kundu 1 , Gopal Pugazhenthi 1 , Tamal Banerjee 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.energyfuels.0c01896 Debashis Kundu 1 , Gopal Pugazhenthi 1 , Tamal Banerjee 1
Affiliation
|
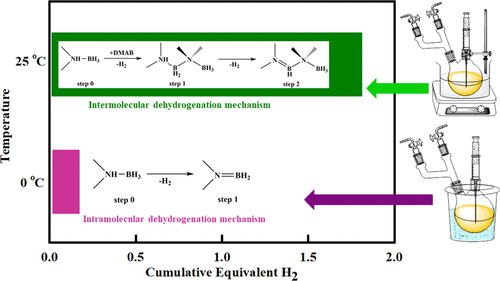
A novel low- and room-temperature dehydrogenation of dimethylamine borane (DMAB) is being reported using hydrogen sulfate-based ionic liquids (ILs), namely, 1-ethyl-3-methylimidazolium hydrogen sulfate ([EMIM][HSO4]) and 1-butyl-3-methylimidazolium hydrogen sulfate ([BMIM][HSO4]). The ILs are selected from a pool of potential ILs using a conductor-like screening model-segment activity coefficient (COSMO-SAC) model, and the dehydrogenation is performed at 0, 15, and 25 °C and a high vacuum of 4 × 10–2 mbar gauge pressure. The dehydrogenated products are characterized with 1H and 11B NMR. 1H NMR characterization reveals the structural integrity of ILs after the dehydrogenation. The 11B NMR characterization reveals the evolution of −BH2 and B═N moieties during dehydrogenation. Based on the 11B NMR study, the IL-assisted intramolecular and intermolecular dehydrogenation mechanism has been proposed. The intramolecular dehydrogenation pathway of dehydrogenation is proposed at 0 °C, and the intermolecular dehydrogenation pathway is at 15 and 25 °C. Formation of DMAB dimers has been confirmed for the intermolecular dehydrogenation pathway; on the contrary, DMAB does not oligomerize as other amine boranes. The interaction energy calculations reveal a greater stability of DMAB in [EMIM][HSO4] than in [BMIM][HSO4] IL. The anionic moiety of ILs is found to be the prominent pathway for the hydrogen bond interaction with protic and hydridic moieties of DMAB. It confirms the dominant role with respect to the selection of anion in hydrogen production.
中文翻译:

离子液体促进的二甲胺硼烷的低温至室温脱氢:分子建模和实验研究
据报道,使用基于硫酸氢的离子液体(ILs),即1-乙基-3-甲基咪唑硫酸氢盐([EMIM] [HSO 4 ])和二甲胺硼烷(DMAs)在室温和室温下进行新型脱氢。1-丁基-3-甲基咪唑硫酸氢盐([BMIM] [HSO 4 ])。使用类似导体的筛选模型-段活度系数(COSMO-SAC)模型从潜在的IL池中选择IL,然后在0、15和25°C以及4×10的高真空下进行脱氢–2 mbar表压。脱氢产物的特征在于1 H和11 B NMR。1 H NMR表征揭示了脱氢后IL的结构完整性。的11 B NMR表征揭示了脱氢过程中-BH 2和B═N部分的演变。基于11 B NMR研究,提出了IL辅助的分子内和分子间脱氢机理。提出了分子内的脱氢途径为0℃,分子间的脱氢途径为15℃和25℃。分子间脱氢途径已经证实了DMAB二聚体的形成。相反,DMAB不会像其他胺硼烷一样低聚。相互作用能计算表明,[EMIM] [HSO 4 ]中的DMAB稳定性比[BMIM] [HSO 4 ]中的DMAB高。] IL。发现ILs的阴离子部分是与DMAB的质子和亲水部分进行氢键相互作用的主要途径。它证实了在制氢中阴离子选择方面的主导作用。
更新日期:2020-10-16
中文翻译:

离子液体促进的二甲胺硼烷的低温至室温脱氢:分子建模和实验研究
据报道,使用基于硫酸氢的离子液体(ILs),即1-乙基-3-甲基咪唑硫酸氢盐([EMIM] [HSO 4 ])和二甲胺硼烷(DMAs)在室温和室温下进行新型脱氢。1-丁基-3-甲基咪唑硫酸氢盐([BMIM] [HSO 4 ])。使用类似导体的筛选模型-段活度系数(COSMO-SAC)模型从潜在的IL池中选择IL,然后在0、15和25°C以及4×10的高真空下进行脱氢–2 mbar表压。脱氢产物的特征在于1 H和11 B NMR。1 H NMR表征揭示了脱氢后IL的结构完整性。的11 B NMR表征揭示了脱氢过程中-BH 2和B═N部分的演变。基于11 B NMR研究,提出了IL辅助的分子内和分子间脱氢机理。提出了分子内的脱氢途径为0℃,分子间的脱氢途径为15℃和25℃。分子间脱氢途径已经证实了DMAB二聚体的形成。相反,DMAB不会像其他胺硼烷一样低聚。相互作用能计算表明,[EMIM] [HSO 4 ]中的DMAB稳定性比[BMIM] [HSO 4 ]中的DMAB高。] IL。发现ILs的阴离子部分是与DMAB的质子和亲水部分进行氢键相互作用的主要途径。它证实了在制氢中阴离子选择方面的主导作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号