当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Efficient Co-Based Metal–Organic Framework Nanocrystal (Co-ZIF-67) for Adsorptive Desulfurization of Dibenzothiophene: Impact of the Preparation Approach on Structure Tuning
Energy & Fuels ( IF 5.3 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.energyfuels.0c01888 Mitra Jafarinasab 1 , Azam Akbari 1 , Mohammadreza Omidkhah 2 , Mozaffar Shakeri 1
Energy & Fuels ( IF 5.3 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.energyfuels.0c01888 Mitra Jafarinasab 1 , Azam Akbari 1 , Mohammadreza Omidkhah 2 , Mozaffar Shakeri 1
Affiliation
|
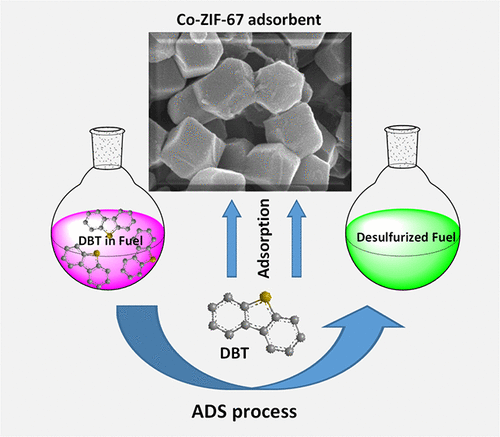
Metal–organic frameworks (MOFs) have attracted considerable attention for selective deep removal of refractory sulfur compounds from fuels. In this work, cobalt-based MOFs (nanocrystals of Co-ZIF-67) were successfully prepared to find a capable adsorbent for removal of dibenzothiophene (DBT) from fuel. This paper revealed the main importance of preparation conditions in adjusting the surface area and pore structure of Co-ZIF-67 crystals for DBT adsorption purposes. Several methods including XRD, SEM, TEM, FTIR, TGA, BET, BJH, and N2 adsorption–desorption were used for characterization of the prepared adsorbents. Screening the adsorbents demonstrated the proper structure and performance of Co-ZIF-67/M nanocrystals synthesized by methanol at room temperature. A considerable promotion in DBT adsorption ability was further reached through exposing more active centers by surface activation. By employing activated Co-ZIF-67/M-A1 (SBET = 2478.8 m2/g), a maximum adsorption capacity of 550 mg/g was attained at T = 65 °C and Madsorbent/Vfuel = 0.8 mg/mL, with an excellent reusability of the adsorbent in four repeated uses. The endothermic nature of DBT adsorption was verified by the positive influence of temperature. A good fitting of experimental data with Langmuir–Freundlich isotherm expressed a possible combination of monolayer and multilayer adsorption of DBT. The kinetic and mechanism studies were also conducted, and possible adsorption pathways were suggested.
中文翻译:

一种高效的基于钴的金属-有机骨架纳米晶体(Co-ZIF-67),用于二苯并噻吩的吸附脱硫:制备方法对结构调整的影响
金属有机框架(MOF)在从燃料中选择性地深度去除耐火硫化合物方面引起了极大的关注。在这项工作中,成功制备了钴基MOF(Co-ZIF-67的纳米晶体),以找到一种能从燃料中去除二苯并噻吩(DBT)的有效吸附剂。本文揭示了制备条件对于调节DBT吸附目的Co-ZIF-67晶体的表面积和孔结构的重要性。几种方法,包括XRD,SEM,TEM,FTIR,TGA,BET,BJH和N 2吸附-解吸用于表征所制备的吸附剂。筛选吸附剂证明了在室温下甲醇合成的Co-ZIF-67 / M纳米晶体的适当结构和性能。通过表面活化暴露更多的活性中心,进一步提高了DBT吸附能力。通过使用活化的Co-ZIF-67 / M-A1(S BET = 2478.8 m 2 / g),在T = 65°C和M吸附剂/ V燃料时,最大吸附容量为550 mg / g。= 0.8 mg / mL,在四次重复使用中具有极好的吸附剂重用性。温度的积极影响证实了DBT吸附的吸热特性。Langmuir-Freundlich等温线的实验数据很好拟合,表明DBT的单层吸附和多层吸附是可能的组合。还进行了动力学和机理研究,并提出了可能的吸附途径。
更新日期:2020-10-16
中文翻译:

一种高效的基于钴的金属-有机骨架纳米晶体(Co-ZIF-67),用于二苯并噻吩的吸附脱硫:制备方法对结构调整的影响
金属有机框架(MOF)在从燃料中选择性地深度去除耐火硫化合物方面引起了极大的关注。在这项工作中,成功制备了钴基MOF(Co-ZIF-67的纳米晶体),以找到一种能从燃料中去除二苯并噻吩(DBT)的有效吸附剂。本文揭示了制备条件对于调节DBT吸附目的Co-ZIF-67晶体的表面积和孔结构的重要性。几种方法,包括XRD,SEM,TEM,FTIR,TGA,BET,BJH和N 2吸附-解吸用于表征所制备的吸附剂。筛选吸附剂证明了在室温下甲醇合成的Co-ZIF-67 / M纳米晶体的适当结构和性能。通过表面活化暴露更多的活性中心,进一步提高了DBT吸附能力。通过使用活化的Co-ZIF-67 / M-A1(S BET = 2478.8 m 2 / g),在T = 65°C和M吸附剂/ V燃料时,最大吸附容量为550 mg / g。= 0.8 mg / mL,在四次重复使用中具有极好的吸附剂重用性。温度的积极影响证实了DBT吸附的吸热特性。Langmuir-Freundlich等温线的实验数据很好拟合,表明DBT的单层吸附和多层吸附是可能的组合。还进行了动力学和机理研究,并提出了可能的吸附途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号