当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The aggregation kinetics and filament structure of a tau fragment are influenced by the sulfation pattern of the cofactor heparin.
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.biochem.0c00443 David Townsend 1 , Nigel J Fullwood 2 , Edwin A Yates 3 , David A Middleton 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-21 , DOI: 10.1021/acs.biochem.0c00443 David Townsend 1 , Nigel J Fullwood 2 , Edwin A Yates 3 , David A Middleton 1
Affiliation
|
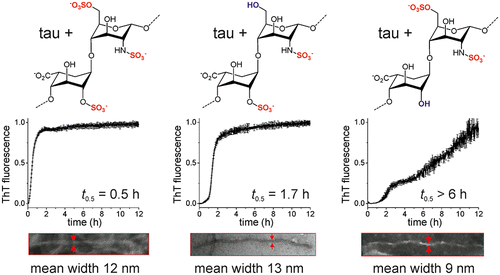
A pathological signature of Alzheimer’s disease (AD) is the formation of neurofibrillary tangles comprising filamentous aggregates of the microtubule associated protein tau. Tau self-assembly is accelerated by polyanions including heparin, an analogue of heparan sulfate. Tau filaments colocalize with heparan sulfate proteoglycans (HSPGs) in vivo, and HSPGs may also assist the transcellular propagation of tau aggregates. Here, we investigate the role of the sulfate moieties of heparin in the aggregation of a recombinant tau fragment Δtau187, comprising residues 255–441 of the C-terminal microtubule-binding domain. The effects that the selective removal of the N-, 2-O-, and 6-O-sulfate groups from heparin have on the kinetics of tau aggregation, aggregate morphology, and protein structure and dynamics were examined. Aggregation kinetics monitored by thioflavin T (ThT) fluorescence revealed that aggregation is considerably slower in the presence of 2-O-desulfated heparin than with N- or 6-O-desulfated heparin. Transmission electron microscopy revealed that tau filaments induced by 2-O-desulfated heparin were more slender than filaments formed in the presence of intact heparin or 6-O-desulfated heparin. The 2-O-desulfated heparin-induced filaments had more extensive regions of flexibility than the other filaments, according to circular dichroism and solid-state NMR spectroscopy. These results indicate that the sulfation pattern of heparin regulates tau aggregation, not purely though electrostatic forces but also through conformational perturbations of heparin when the 2-O-sulfate is removed. These findings may have implications for the progression of AD, as the sulfation pattern of GAGs is known to change during the aging process, which is the main risk factor for the disease.
中文翻译:

tau 片段的聚集动力学和丝结构受辅因子肝素的硫酸化模式影响。
阿尔茨海默氏病 (AD) 的病理特征是形成包含微管相关蛋白 tau 的丝状聚集体的神经原纤维缠结。 Tau 自组装可通过聚阴离子加速,包括肝素(硫酸乙酰肝素的类似物)。 Tau 丝在体内与硫酸乙酰肝素蛋白聚糖 (HSPG) 共定位,HSPG 也可能有助于 tau 聚集体的跨细胞传播。在这里,我们研究了肝素硫酸盐部分在重组 tau 片段 Δtau187 聚集中的作用,该片段包含 C 端微管结合域的残基 255-441。研究了从肝素中选择性去除N -、2- O - 和 6- O -硫酸盐基团对 tau 聚集动力学、聚集体形态以及蛋白质结构和动力学的影响。通过硫黄素 T (ThT) 荧光监测的聚集动力学表明,在 2- O-脱硫肝素存在下,聚集速度比在N-或 6- O-脱硫肝素存在下慢得多。透射电子显微镜显示,由 2- O-脱硫肝素诱导的 tau 丝比在完整肝素或 6- O-脱硫肝素存在下形成的丝更细长。根据圆二色性和固态核磁共振光谱,2- O-脱硫肝素诱导的细丝比其他细丝具有更广泛的柔性区域。这些结果表明,肝素的硫酸化模式不仅通过静电力,而且还通过去除 2- O-硫酸盐时肝素的构象扰动来调节 tau 聚集。 这些发现可能对 AD 的进展有影响,因为已知 GAG 的硫酸化模式会在衰老过程中发生变化,这是该疾病的主要危险因素。
更新日期:2020-10-21
中文翻译:

tau 片段的聚集动力学和丝结构受辅因子肝素的硫酸化模式影响。
阿尔茨海默氏病 (AD) 的病理特征是形成包含微管相关蛋白 tau 的丝状聚集体的神经原纤维缠结。 Tau 自组装可通过聚阴离子加速,包括肝素(硫酸乙酰肝素的类似物)。 Tau 丝在体内与硫酸乙酰肝素蛋白聚糖 (HSPG) 共定位,HSPG 也可能有助于 tau 聚集体的跨细胞传播。在这里,我们研究了肝素硫酸盐部分在重组 tau 片段 Δtau187 聚集中的作用,该片段包含 C 端微管结合域的残基 255-441。研究了从肝素中选择性去除N -、2- O - 和 6- O -硫酸盐基团对 tau 聚集动力学、聚集体形态以及蛋白质结构和动力学的影响。通过硫黄素 T (ThT) 荧光监测的聚集动力学表明,在 2- O-脱硫肝素存在下,聚集速度比在N-或 6- O-脱硫肝素存在下慢得多。透射电子显微镜显示,由 2- O-脱硫肝素诱导的 tau 丝比在完整肝素或 6- O-脱硫肝素存在下形成的丝更细长。根据圆二色性和固态核磁共振光谱,2- O-脱硫肝素诱导的细丝比其他细丝具有更广泛的柔性区域。这些结果表明,肝素的硫酸化模式不仅通过静电力,而且还通过去除 2- O-硫酸盐时肝素的构象扰动来调节 tau 聚集。 这些发现可能对 AD 的进展有影响,因为已知 GAG 的硫酸化模式会在衰老过程中发生变化,这是该疾病的主要危险因素。










































 京公网安备 11010802027423号
京公网安备 11010802027423号