当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Addition of 2‐Halogenopyridines and 2‐Chloroquinoline to Zero‐Valent Group 10 Metals
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-09-21 , DOI: 10.1002/zaac.202000279 Mareike C. Jahnke 1 , Ramona M. C. Pichl 1 , F. Ekkehardt Hahn 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-09-21 , DOI: 10.1002/zaac.202000279 Mareike C. Jahnke 1 , Ramona M. C. Pichl 1 , F. Ekkehardt Hahn 1
Affiliation
|
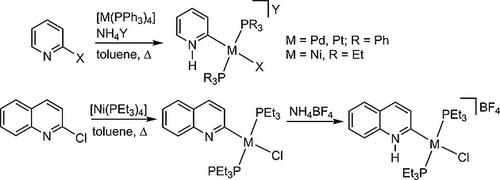
Oxidative addition of the C2–X (X = Cl, Br) bond of 2‐halogenopyridines or 2‐chloroquinoline to zero‐valent group 10 metal complexes in the presence of a proton source leads to complexes bearing pyridine‐derived protic remote NHC (rNHC) ligands. The oxidative addition of 2‐halogeno‐functionalized pyridine derivatives to complexes [M0(PPh3)4] (M = Pd, Pt) in the presence of NH4BF4 or NH4PF6 yielded complexes trans‐[1]PF6–trans‐[5]BF4 with a trans‐arrangement of the two remaining PPh3 ligands. The oxidative addition to Ni0 was achieved by reaction of the halogenopyridine derivatives with [Ni(cod)2]/PEt3 in the presence of a proton acid (NH4BF4 or NH4PF6) yielding complexes of type trans‐[Ni(protic‐rNHC)(PEt3)2X]A (A = BF4–, PF6–). In the absence of a proton source, the oxidative addition of 2‐chloroquinone to [Ni(cod)2]/PEt3 yielded the neutral complex trans‐[8] bearing an anionic quinoline‐derived rNHC ligand. The molecular structures of selected complexes have been determined by X‐ray diffraction studies.
中文翻译:

2-卤代吡啶和2-氯喹啉向零价10族金属的氧化加成
在质子源的存在下,将2-卤代吡啶或2-氯喹啉的C2-X(X = Cl,Br)键氧化成零价第10组金属配合物,导致配合物带有吡啶衍生的质子远程NHC(r NHC)配体。在NH 4 BF 4或NH 4 PF 6存在下,将2-卤代官能化吡啶衍生物氧化成配合物[M 0(PPh 3)4 ](M = Pd,Pt),得到反式-[ 1 ] PF的配合物6 –反式[[ 5 ] BF 4带反式剩余的两个PPh 3配体的排列。通过在质子酸(NH 4 BF 4或NH 4 PF 6)存在下使卤代吡啶衍生物与[Ni(cod)2 ] / PEt 3反应,可实现Ni 0的氧化加成反应,生成反式-[的Ni(protic- ř NHC)(PET 3)2 X] A(A = BF 4 -,PF 6 - )。在没有质子源的情况下,将2-氯醌氧化加入[Ni(cod)2 ] / PEt 3中生成带有阴离子喹啉衍生的r NHC配体的中性复合物反式[[ 8 ] 。所选配合物的分子结构已通过X射线衍射研究确定。
更新日期:2020-09-21
中文翻译:

2-卤代吡啶和2-氯喹啉向零价10族金属的氧化加成
在质子源的存在下,将2-卤代吡啶或2-氯喹啉的C2-X(X = Cl,Br)键氧化成零价第10组金属配合物,导致配合物带有吡啶衍生的质子远程NHC(r NHC)配体。在NH 4 BF 4或NH 4 PF 6存在下,将2-卤代官能化吡啶衍生物氧化成配合物[M 0(PPh 3)4 ](M = Pd,Pt),得到反式-[ 1 ] PF的配合物6 –反式[[ 5 ] BF 4带反式剩余的两个PPh 3配体的排列。通过在质子酸(NH 4 BF 4或NH 4 PF 6)存在下使卤代吡啶衍生物与[Ni(cod)2 ] / PEt 3反应,可实现Ni 0的氧化加成反应,生成反式-[的Ni(protic- ř NHC)(PET 3)2 X] A(A = BF 4 -,PF 6 - )。在没有质子源的情况下,将2-氯醌氧化加入[Ni(cod)2 ] / PEt 3中生成带有阴离子喹啉衍生的r NHC配体的中性复合物反式[[ 8 ] 。所选配合物的分子结构已通过X射线衍射研究确定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号