Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Hybrid Organo-Nanotheranostic Platform of Superlative Biocompatibility for Near-Infrared-Triggered Fluorescence Imaging and Synergistically Enhanced Ablation of Tumors.
Small ( IF 13.0 ) Pub Date : 2020-09-20 , DOI: 10.1002/smll.202002445 Ozioma Udochukwu Akakuru 1, 2 , Chuang Liu 1, 2 , M Zubair Iqbal 1, 3 , Gohar Ijaz Dar 1, 2 , Gao Yang 1 , Kun Qian 1, 2 , Elvis Ikechukwu Nosike 1, 2 , Jie Xing 1, 2 , Zhoujing Zhang 1 , Yanying Li 1, 2 , Juan Li 1 , Aiguo Wu 1
Small ( IF 13.0 ) Pub Date : 2020-09-20 , DOI: 10.1002/smll.202002445 Ozioma Udochukwu Akakuru 1, 2 , Chuang Liu 1, 2 , M Zubair Iqbal 1, 3 , Gohar Ijaz Dar 1, 2 , Gao Yang 1 , Kun Qian 1, 2 , Elvis Ikechukwu Nosike 1, 2 , Jie Xing 1, 2 , Zhoujing Zhang 1 , Yanying Li 1, 2 , Juan Li 1 , Aiguo Wu 1
Affiliation
|
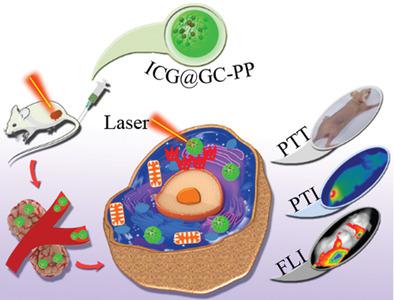
The quest for an all‐organic nanosystem with negligible cytotoxicity and remarkable in vivo tumor theranostic capability is inescapably unending. Hitherto, the landscape of available photothermal agents is dominated by metal‐based nanoparticles (NPs) with attendant in vivo negatives. Here, an all‐organic‐composed theranostic nanosystem with outstanding biocompatibility for fluorescence image‐guided tumor photothermal therapy, and as a potential alternative to metal‐based photothermal agents is developed. This is rationally achieved by compartmentalizing indocyanine green (ICG) in glycol chitosan (GC)‐polypyrrole (PP) nanocarrier to form hybrid ICG@GC‐PP NPs (≈65 nm). The compartmentalization strategy, alongside the high photothermal conversion ability of PP jointly enhances the low photostability of free ICG. Advantageously, ICG@GC‐PP is endowed with an impeccable in vivo performance by the well‐known biocompatibility track records of its individual tri organo‐components (GC, PP, and ICG). As a proof of concept, ICG@GC‐PP NPs enables a sufficiently prolonged tumor diagnosis by fluorescence imaging up to 20 h post‐injection. Furthermore, owing to the complementary heating performances of PP and ICG, ICG@GC‐PP NPs‐treated mice by one‐time near‐infrared irradiation exhibit total tumor regression within 14 days post‐treatment. Therefore, leveraging the underlying benefits of this study will help to guide the development of new all‐organic biocompatible systems in synergism, for safer tumor theranostics.
中文翻译:

最佳近红外触发荧光成像和协同增强消融的最高级生物相容性的混合有机纳米技术平台。
对具有可忽略的细胞毒性和卓越的体内肿瘤治疗能力的全有机纳米系统的追求是不可避免的。迄今为止,可用的光热剂的领域主要是金属基纳米颗粒(NP)和伴随的体内阴性。在这里,开发了一种具有出色生物相容性的全有机组成的治疗药物纳米系统,可用于荧光图像引导的肿瘤光热疗法,并可以替代金属基光热剂。通过在乙二醇壳聚糖(GC)-聚吡咯(PP)纳米载体中分隔吲哚菁绿(ICG)以形成杂化ICG @ GC-PP NP(≈65nm),可以合理地实现这一目标。隔室化策略以及PP的高光热转化能力共同增强了游离ICG的低光稳定性。有利地,ICG @ GC‐PP的体内三有机组分(GC,PP和ICG)的众所周知的生物相容性跟踪记录赋予其无可挑剔的体内性能。作为概念的证明,ICG @ GC-PP NP可以在注射后20小时内通过荧光成像充分延长肿瘤的诊断时间。此外,由于PP和ICG具有互补的加热性能,ICG @ GC‐PP NPs一次性经近红外辐射处理的小鼠在治疗后14天内表现出肿瘤完全消退。因此,利用本研究的潜在益处将有助于指导新的全有机生物相容系统的协同增效作用,以实现更安全的肿瘤治疗学。ICG @ GC‐PP NP可以在注射后20小时内通过荧光成像充分延长肿瘤的诊断时间。此外,由于PP和ICG具有互补的加热性能,ICG @ GC‐PP NPs一次性经近红外辐射处理的小鼠在治疗后14天内表现出肿瘤完全消退。因此,利用本研究的潜在益处将有助于指导新的全有机生物相容系统的协同增效作用,以实现更安全的肿瘤治疗学。ICG @ GC-PP NPs可以在注射后20小时内通过荧光成像充分延长肿瘤的诊断时间。此外,由于PP和ICG具有互补的加热性能,ICG @ GC‐PP NPs一次性经近红外辐射处理的小鼠在治疗后14天内表现出肿瘤完全消退。因此,利用本研究的潜在益处将有助于指导新的全有机生物相容系统的协同增效作用,以实现更安全的肿瘤治疗学。
更新日期:2020-10-16
中文翻译:

最佳近红外触发荧光成像和协同增强消融的最高级生物相容性的混合有机纳米技术平台。
对具有可忽略的细胞毒性和卓越的体内肿瘤治疗能力的全有机纳米系统的追求是不可避免的。迄今为止,可用的光热剂的领域主要是金属基纳米颗粒(NP)和伴随的体内阴性。在这里,开发了一种具有出色生物相容性的全有机组成的治疗药物纳米系统,可用于荧光图像引导的肿瘤光热疗法,并可以替代金属基光热剂。通过在乙二醇壳聚糖(GC)-聚吡咯(PP)纳米载体中分隔吲哚菁绿(ICG)以形成杂化ICG @ GC-PP NP(≈65nm),可以合理地实现这一目标。隔室化策略以及PP的高光热转化能力共同增强了游离ICG的低光稳定性。有利地,ICG @ GC‐PP的体内三有机组分(GC,PP和ICG)的众所周知的生物相容性跟踪记录赋予其无可挑剔的体内性能。作为概念的证明,ICG @ GC-PP NP可以在注射后20小时内通过荧光成像充分延长肿瘤的诊断时间。此外,由于PP和ICG具有互补的加热性能,ICG @ GC‐PP NPs一次性经近红外辐射处理的小鼠在治疗后14天内表现出肿瘤完全消退。因此,利用本研究的潜在益处将有助于指导新的全有机生物相容系统的协同增效作用,以实现更安全的肿瘤治疗学。ICG @ GC‐PP NP可以在注射后20小时内通过荧光成像充分延长肿瘤的诊断时间。此外,由于PP和ICG具有互补的加热性能,ICG @ GC‐PP NPs一次性经近红外辐射处理的小鼠在治疗后14天内表现出肿瘤完全消退。因此,利用本研究的潜在益处将有助于指导新的全有机生物相容系统的协同增效作用,以实现更安全的肿瘤治疗学。ICG @ GC-PP NPs可以在注射后20小时内通过荧光成像充分延长肿瘤的诊断时间。此外,由于PP和ICG具有互补的加热性能,ICG @ GC‐PP NPs一次性经近红外辐射处理的小鼠在治疗后14天内表现出肿瘤完全消退。因此,利用本研究的潜在益处将有助于指导新的全有机生物相容系统的协同增效作用,以实现更安全的肿瘤治疗学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号