当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, antimicrobial screening, and docking study of new 2‐(2‐ethylpyridin‐4‐yl)‐4‐methyl‐N‐phenylthiazole‐5‐carboxamide derivatives
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-09-21 , DOI: 10.1002/jccs.202000174 Sanghratna L. Kasare 1 , Pornima N. Gund 1 , Bhaurao P. Sathe 1 , Pravin S. Patil 1 , Naziya N. M. A. Rehman 2 , Prashant P. Dixit 2 , Prafulla B. Choudhari 3 , Kishan P. Haval 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-09-21 , DOI: 10.1002/jccs.202000174 Sanghratna L. Kasare 1 , Pornima N. Gund 1 , Bhaurao P. Sathe 1 , Pravin S. Patil 1 , Naziya N. M. A. Rehman 2 , Prashant P. Dixit 2 , Prafulla B. Choudhari 3 , Kishan P. Haval 1
Affiliation
|
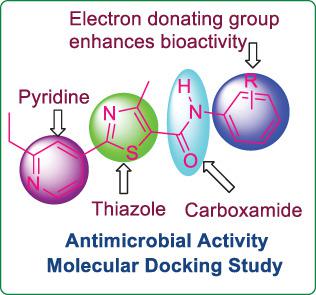
A series of new 2‐(2‐ethylpyridin‐4‐yl)‐4‐methyl‐N‐phenylthiazole‐5‐carboxamide derivatives (5a‐l) were synthesized and evaluated for their in vitro antimicrobial activities. Among the screened compounds, 5b, 5d, 5e, 5f, and 5j have shown promising antimicrobial activities against both bacterial and fungal pathogens. A molecular docking study was conducted to know the probable mode of action of synthesized derivatives for antimicrobial activity. The active compounds have shown excellent binding affinity toward DNA gyrase and lumazine synthase enzymes. The physicochemical properties of the synthesized thiazole‐carboxamide derivatives were calculated. It has displayed the potential to be a reasonable oral bioavailability drug as determined by Lipinski's rule.
中文翻译:

新的2-(2-乙基吡啶-4-基)-4-甲基-N-苯基噻唑-5-羧酰胺衍生物的合成,抗菌筛选和对接研究
合成了一系列新的2-(2-乙基吡啶-4-基)-4-甲基-N-苯基噻唑-5-羧酰胺衍生物(5a-1)并评估了它们的体外抗菌活性。在筛选的化合物中,5b,5d,5e,5f和5j显示出对细菌和真菌病原体都有希望的抗菌活性。进行了分子对接研究,以了解合成衍生物对抗菌活性的可能作用方式。活性化合物对DNA促旋酶和lumazine合酶显示出极好的结合亲和力酶。计算了合成的噻唑-羧酰胺衍生物的理化性质。根据Lipinski法则,它已显示出成为合理的口服生物利用度药物的潜力。
更新日期:2020-09-21
中文翻译:

新的2-(2-乙基吡啶-4-基)-4-甲基-N-苯基噻唑-5-羧酰胺衍生物的合成,抗菌筛选和对接研究
合成了一系列新的2-(2-乙基吡啶-4-基)-4-甲基-N-苯基噻唑-5-羧酰胺衍生物(5a-1)并评估了它们的体外抗菌活性。在筛选的化合物中,5b,5d,5e,5f和5j显示出对细菌和真菌病原体都有希望的抗菌活性。进行了分子对接研究,以了解合成衍生物对抗菌活性的可能作用方式。活性化合物对DNA促旋酶和lumazine合酶显示出极好的结合亲和力酶。计算了合成的噻唑-羧酰胺衍生物的理化性质。根据Lipinski法则,它已显示出成为合理的口服生物利用度药物的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号