Brain Research ( IF 2.7 ) Pub Date : 2020-09-21 , DOI: 10.1016/j.brainres.2020.147117 Mariana Grigoruta 1 , Marbella Chavez-Solano 1 , Armando Varela-Ramirez 2 , Jorge A Sierra-Fonseca 2 , Ernesto Orozco-Lucero 3 , Jameel N Hamdan 2 , Kristin L Gosselink 2 , Alejandro Martinez-Martinez 4
|
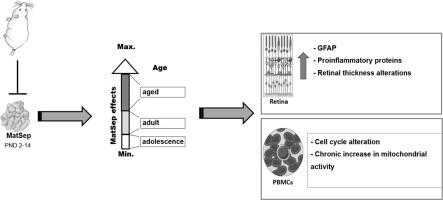
Early life stress alters the function and feedback regulation of the hypothalamic-pituitaryadrenal (HPA) axis, and can contribute to neuroinflammation and neurodegeneration by modifying peripheral blood mononuclear cell (PBMC) activity. The retina, as part of the nervous system, is sensitive to immune changes induced by stress. However, the consequences of stress experienced at an early age on retinal development have not yet been elucidated. Here we aimed to evaluate the impact of maternal separation (MatSep) across three stages of the lifespan (adolescent, adult, and aged) on the retina, as well as on progression through the cell cycle and mitochondrial activity in PBMCs from female Wistar rats. Newborn pups were separated from their mother from postnatal day (PND) 2 until PND 14 for 3 h/day. Retinal analysis from the MatSep groups showed architectural alterations such as a diminished thickness of retinal layers, as well as increased expression of proinflammatory markers DJ-1, Iba-1, and CD45 and the gliotic marker GFAP. Additionally, MatSep disrupted the cell cycle and caused long-term increases in mitochondrial activity in PBMCs from adolescent and adult rats. Changes in the cell cycle profile of the PBMCs from aged MatSep rats were undetected. However, these PBMCs exhibited increased sensitivity to H2O2-induced oxidative stress in vitro. Therefore, these results suggest that early life stress can have long-term effects on retinal structure and function, possibly elicited by neonatal immune preconditioning.
中文翻译:

母体分离诱导雌性大鼠整个生命周期的视网膜和外周血单个核细胞改变
早期生活压力会改变下丘脑 - 垂体肾上腺 (HPA) 轴的功能和反馈调节,并且可以通过改变外周血单核细胞 (PBMC) 活性来促进神经炎症和神经变性。作为神经系统的一部分,视网膜对压力引起的免疫变化很敏感。然而,尚未阐明早年经历的压力对视网膜发育的影响。在这里,我们旨在评估母体分离 (MatSep) 在生命周期的三个阶段(青春期、成人和老年)对视网膜的影响,以及对雌性 Wistar 大鼠 PBMC 中细胞周期和线粒体活动的影响。新生幼崽从出生后第 2 天 (PND) 到第 14 天与母亲分开 3 小时/天。MatSep 组的视网膜分析显示结构改变,例如视网膜层厚度减少,以及促炎标记物 DJ-1、Iba-1 和 CD45 以及胶质细胞标记物 GFAP 的表达增加。此外,MatSep 扰乱了细胞周期,并导致青春期和成年大鼠 PBMC 中线粒体活性的长期增加。未检测到来自老年 MatSep 大鼠的 PBMC 的细胞周期特征的变化。然而,这些 PBMC 对 H 表现出更高的敏感性 未检测到来自老年 MatSep 大鼠的 PBMC 的细胞周期特征的变化。然而,这些 PBMC 对 H 表现出更高的敏感性 未检测到来自老年 MatSep 大鼠的 PBMC 的细胞周期特征的变化。然而,这些 PBMC 对 H 表现出更高的敏感性2 O 2诱导的体外氧化应激。因此,这些结果表明,早期生活压力会对视网膜结构和功能产生长期影响,这可能是由新生儿免疫预处理引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号