当前位置:
X-MOL 学术
›
Appl. Geochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Main formation conditions of soda-type groundwater: a case study from south-eastern Transbaikal region (Russia)
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.apgeochem.2020.104763 Svetlana V. Borzenko , Valeriia V. Drebot , Igor A. Fedorov
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.apgeochem.2020.104763 Svetlana V. Borzenko , Valeriia V. Drebot , Igor A. Fedorov
|
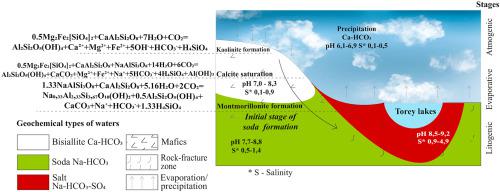
Abstract Chemical and isotopic groundwater composition analyses in the south-eastern Transbaikal case study region allowed three geochemical types of waters and fifteen subtypes to be identified: bisiallite (Ca–HCO3), soda (Na–HCO3) and salt (Na–HCO3–Cl–SO4). All three types differed significantly with respect to TDS content, pH, composition of chemical elements and heavy water isotopes. The transition from the first to the subsequent types was accompanied by CO32−, HCO3−, SO42−, Cl− and Na+ accumulation, whereas a weaker dependence was exhibited for Mg2+ and K+, and no relationship with Ca2+ was found. Water was found to be gradually enriched in heavy isotopes and some trace elements (e.g. F, Mo, Br, B and Li). Findings showed that the groundwater–rock system is one of an equilibrium-disequilibrium. From the extent of the deeper horizons to the surface water, groundwater in the study region continuously dissolves minerals that are in disequilibrium with the groundwater and simultaneously forms new minerals. Moreover, over time, not only does the composition of the solution change, but the type of secondary minerals and the ratio between chemical elements also changes because some elements precipitate out of solution, while others continue to accumulate. The stage of soda-type groundwater formation was found to be at an intermediate level between the formation of fresh bisiallite (Ca–HCO3) water of different cationic composition and salt (Na–HCO3–Cl–SO4) water with mixed anionic composition.
中文翻译:

苏打型地下水的主要形成条件——以外贝加尔湖东南部地区(俄罗斯)为例
摘要 外贝加尔湖东南部案例研究区的化学和同位素地下水成分分析允许确定三种地球化学类型的水和 15 个亚型:双铝盐 (Ca-HCO3)、苏打 (Na-HCO3) 和盐 (Na-HCO3-Cl) –SO4)。所有三种类型在 TDS 含量、pH、化学元素组成和重水同位素方面都有显着差异。从第一种类型到后续类型的转变伴随着 CO32-、HCO3-、SO42-、Cl- 和 Na+ 的积累,而对 Mg2+ 和 K+ 的依赖性较弱,并且与 Ca2+ 没有关系。发现水中逐渐富含重同位素和一些微量元素(如 F、Mo、Br、B 和 Li)。研究结果表明,地下水-岩石系统是一种平衡-不平衡系统。从更深的地层到地表水,研究区地下水不断溶解与地下水不平衡的矿物质,同时形成新的矿物质。此外,随着时间的推移,不仅溶液的成分发生变化,次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。研究区的地下水不断溶解与地下水不平衡的矿物质,同时形成新的矿物质。此外,随着时间的推移,不仅溶液的成分发生变化,次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。研究区的地下水不断溶解与地下水不平衡的矿物质,同时形成新的矿物质。此外,随着时间的推移,不仅溶液的成分发生变化,次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。但是次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。但是次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。
更新日期:2020-12-01
中文翻译:

苏打型地下水的主要形成条件——以外贝加尔湖东南部地区(俄罗斯)为例
摘要 外贝加尔湖东南部案例研究区的化学和同位素地下水成分分析允许确定三种地球化学类型的水和 15 个亚型:双铝盐 (Ca-HCO3)、苏打 (Na-HCO3) 和盐 (Na-HCO3-Cl) –SO4)。所有三种类型在 TDS 含量、pH、化学元素组成和重水同位素方面都有显着差异。从第一种类型到后续类型的转变伴随着 CO32-、HCO3-、SO42-、Cl- 和 Na+ 的积累,而对 Mg2+ 和 K+ 的依赖性较弱,并且与 Ca2+ 没有关系。发现水中逐渐富含重同位素和一些微量元素(如 F、Mo、Br、B 和 Li)。研究结果表明,地下水-岩石系统是一种平衡-不平衡系统。从更深的地层到地表水,研究区地下水不断溶解与地下水不平衡的矿物质,同时形成新的矿物质。此外,随着时间的推移,不仅溶液的成分发生变化,次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。研究区的地下水不断溶解与地下水不平衡的矿物质,同时形成新的矿物质。此外,随着时间的推移,不仅溶液的成分发生变化,次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。研究区的地下水不断溶解与地下水不平衡的矿物质,同时形成新的矿物质。此外,随着时间的推移,不仅溶液的成分发生变化,次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。但是次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。但是次生矿物的类型和化学元素之间的比例也会发生变化,因为一些元素从溶液中沉淀出来,而另一些元素则继续积累。苏打型地下水的形成阶段处于形成不同阳离子组成的新鲜双铝盐 (Ca-HCO3) 水和混合阴离子组成的盐 (Na-HCO3-Cl-SO4) 水之间的中间水平。









































 京公网安备 11010802027423号
京公网安备 11010802027423号