当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Mechanistic Study on the [Au(NHC)]+-Catalyzed Hydration of Alkynes, Alkenes, and Allenes
Organometallics ( IF 2.5 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.organomet.0c00292 Giuseppe Sciortino 1, 2 , Sara Muñoz-López 1 , Agustí Lledós 1 , Gregori Ujaque 1
Organometallics ( IF 2.5 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.organomet.0c00292 Giuseppe Sciortino 1, 2 , Sara Muñoz-López 1 , Agustí Lledós 1 , Gregori Ujaque 1
Affiliation
|
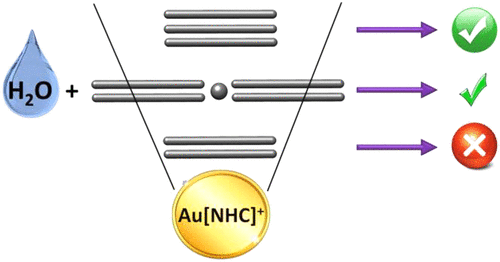
The addition of water molecules to unsaturated substrates is a highly desirable process. Additions to alkynes are very common, whereas additions to allenes and specially alkenes are rather scarce. One of the main aims here is to perform a comparative analysis of their reaction mechanisms for the process catalyzed by Au(I); another objective is to analyze why alkenes are much less reactive than their alkyne or allene counterparts. With this purpose the reaction mechanism for the addition of water to terminal and internal alkynes, alkenes, and allenes catalyzed by an [Au(NHC)]+ complex (NHC = N-heterocyclic carbene) is analyzed by means of DFT calculations. The general catalytic cycle for the three kinds of substrates can be described by three main steps: (i) reactant π coordination to the Au(I) complex, (ii) water nucleophilic addition, and (iii) protodeauration, with subtle differences among the reactants. A comparative analysis, from the evolution of the centroids of localized molecular orbitals (CLMO), of the electronic rearrangements taking place in the protodeauration step reveals different mechanisms for these three substrates, both regarding the electron pair that accepts the proton and the fate of the Au–C bond pair. For alkenes calculations show that nucleophilic addition is highly demanding but affordable, whereas protodeauration is in any case energetically prohibitive. The main reason is not the intrinsic barrier of the protodeauration step, just a few kcal mol–1 higher than that of alkynes, but the high energy of the water-added intermediate. This issue is not related to the strength of the Au(I)–CC bond but to that of the C–O bond.
中文翻译:

[Au(NHC)] +催化炔烃,烯烃和丙二烯水合反应的比较机理研究
将水分子添加到不饱和底物中是非常需要的方法。炔烃的添加非常普遍,而丙二烯和特别是烯烃的添加相当稀少。这里的主要目的之一是对由Au(I)催化的过程的反应机理进行比较分析。另一个目的是分析为什么烯烃的反应性要比炔烃或丙二烯低得多。为此目的,在[Au(NHC)] +催化下,向末端和内部炔烃,烯烃和丙二烯加水的反应机理配合物(NHC = N-杂环卡宾)通过DFT计算进行分析。三种底物的一般催化循环可以通过三个主要步骤来描述:(i)反应物与Au(I)配合物的π配位;(ii)水亲核加成;和(iii)原型脱氢,两者之间存在细微差别反应物。从局部分子轨道的质心演变(CLMO)进行的比较分析,发现原离子去除步骤中发生的电子重排显示出这三种底物的机制不同,这两种情况都与接受质子的电子对和电子的命运有关。 Au–C键对。对于烯烃的计算表明,亲核加成是非常苛刻的,但可负担得起,而原脱氢无论如何在能量上都是禁止的。比炔烃高–1,但加水中间体的能量很高。此问题与Au(I)-CC键的强度无关,而与C-O键的强度无关。
更新日期:2020-10-12
中文翻译:

[Au(NHC)] +催化炔烃,烯烃和丙二烯水合反应的比较机理研究
将水分子添加到不饱和底物中是非常需要的方法。炔烃的添加非常普遍,而丙二烯和特别是烯烃的添加相当稀少。这里的主要目的之一是对由Au(I)催化的过程的反应机理进行比较分析。另一个目的是分析为什么烯烃的反应性要比炔烃或丙二烯低得多。为此目的,在[Au(NHC)] +催化下,向末端和内部炔烃,烯烃和丙二烯加水的反应机理配合物(NHC = N-杂环卡宾)通过DFT计算进行分析。三种底物的一般催化循环可以通过三个主要步骤来描述:(i)反应物与Au(I)配合物的π配位;(ii)水亲核加成;和(iii)原型脱氢,两者之间存在细微差别反应物。从局部分子轨道的质心演变(CLMO)进行的比较分析,发现原离子去除步骤中发生的电子重排显示出这三种底物的机制不同,这两种情况都与接受质子的电子对和电子的命运有关。 Au–C键对。对于烯烃的计算表明,亲核加成是非常苛刻的,但可负担得起,而原脱氢无论如何在能量上都是禁止的。比炔烃高–1,但加水中间体的能量很高。此问题与Au(I)-CC键的强度无关,而与C-O键的强度无关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号