当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Nitrene-Type Rearrangement for the Commercial Route of the JAK1 Inhibitor Abrocitinib
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.oprd.0c00366 Christina G. Connor , Jacob C. DeForest , Phil Dietrich , Nga M. Do , Kevin M. Doyle , Shane Eisenbeis , Elizabeth Greenberg , Sarah H. Griffin , Brian P. Jones , Kris N. Jones , Michael Karmilowicz , Rajesh Kumar , Chad A. Lewis , Emma L. McInturff , J. Christopher McWilliams , Ruchi Mehta , Bao D. Nguyen , Anil M. Rane , Brian Samas , Barbara J. Sitter , Howard W. Ward , Mark E. Webster
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-09-18 , DOI: 10.1021/acs.oprd.0c00366 Christina G. Connor , Jacob C. DeForest , Phil Dietrich , Nga M. Do , Kevin M. Doyle , Shane Eisenbeis , Elizabeth Greenberg , Sarah H. Griffin , Brian P. Jones , Kris N. Jones , Michael Karmilowicz , Rajesh Kumar , Chad A. Lewis , Emma L. McInturff , J. Christopher McWilliams , Ruchi Mehta , Bao D. Nguyen , Anil M. Rane , Brian Samas , Barbara J. Sitter , Howard W. Ward , Mark E. Webster
|
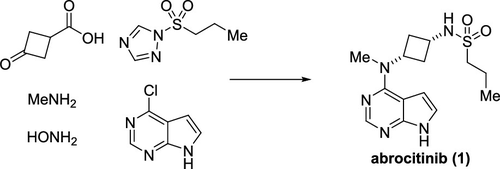
The development of a commercial route toward the JAK1 inhibitor abrocitinib is described. The application of a late-stage Lossen rearrangement provided the desired cis-diaminocyclobutane, which was subsequently sulfonylated using a novel water-tolerable triazole sulfonylating reagent to provide the active pharmaceutical ingredient.
中文翻译:

用于JAK1抑制剂阿布罗替尼商业路线的丁二烯型重排的开发。
描述了朝着JAK1抑制剂阿布罗替尼的商业途径的发展。后期Lossen重排的应用提供了所需的顺式-二氨基环丁烷,随后使用新型的耐水三唑磺酰化试剂将其磺酰化,以提供活性药物成分。
更新日期:2020-09-18
中文翻译:

用于JAK1抑制剂阿布罗替尼商业路线的丁二烯型重排的开发。
描述了朝着JAK1抑制剂阿布罗替尼的商业途径的发展。后期Lossen重排的应用提供了所需的顺式-二氨基环丁烷,随后使用新型的耐水三唑磺酰化试剂将其磺酰化,以提供活性药物成分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号