当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sterically Tuned N-Heterocyclic Carbene Ligands for the Efficient Formation of Hindered Products in Ru-Catalyzed Olefin Metathesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-18 , DOI: 10.1021/acscatal.0c02770 Sebastian Planer 1 , Paweł Małecki 1 , Bartosz Trzaskowski 2 , Anna Kajetanowicz 1 , Karol Grela 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-09-18 , DOI: 10.1021/acscatal.0c02770 Sebastian Planer 1 , Paweł Małecki 1 , Bartosz Trzaskowski 2 , Anna Kajetanowicz 1 , Karol Grela 1
Affiliation
|
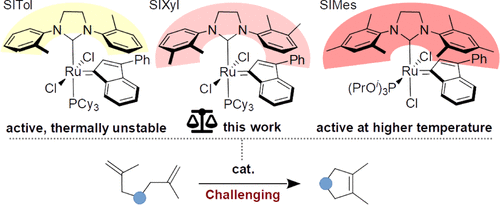
Formation of tetrasubstituted C–C double bonds via olefin metathesis is considered very challenging for classical Ru-based complexes. In the hope to improve this condition, three ruthenium olefin metathesis catalysts bearing sterically reduced N-heterocyclic carbene (NHC) ligands with xylyl “arms” were synthesized, characterized using both computational and experimental techniques, and tested in a number of challenging reactions. The catalysts are predicted to initiate much faster than the analogue with mesityl N-substituents. We also foreboded the rotation of xylyl side groups at ambient temperature and the existence of all four atropoisomers in the solution, which was in agreement with experimental data. These catalysts exhibited high activity at relatively low temperatures (45–60 °C) and at reduced catalyst loadings in various reactions of sterically hindered alkenes, including complex polyfunctional substrates of pharmaceutical interest, such as yangonin precursors, chrysantemic acid derivatives, analogues of cannabinoid agonists, α-terpineol, and finally a thermally unstable peroxide.
中文翻译:

空间调谐的 N-杂环卡宾配体用于在 Ru 催化的烯烃复分解中有效形成受阻产物
通过烯烃复分解形成四取代的 C-C 双键被认为对于经典的钌基配合物非常具有挑战性。为了改善这种情况,合成了三种带有二甲苯基“臂”的空间还原的N-杂环卡宾(NHC)配体的钌烯烃复分解催化剂,使用计算和实验技术进行了表征,并在许多具有挑战性的反应中进行了测试。预计该催化剂的引发速度比带有异丙基N取代基的类似物快得多。我们还预见到二甲苯侧基在环境温度下的旋转以及溶液中所有四种阻转异构体的存在,这与实验数据一致。这些催化剂在相对较低的温度(45-60°C)和减少的催化剂负载量下,在空间位阻烯烃的各种反应中表现出高活性,包括具有制药意义的复杂多功能底物,例如仰光素前体、菊酸衍生物、大麻素激动剂类似物、α-萜品醇,最后是热不稳定的过氧化物。
更新日期:2020-10-02
中文翻译:

空间调谐的 N-杂环卡宾配体用于在 Ru 催化的烯烃复分解中有效形成受阻产物
通过烯烃复分解形成四取代的 C-C 双键被认为对于经典的钌基配合物非常具有挑战性。为了改善这种情况,合成了三种带有二甲苯基“臂”的空间还原的N-杂环卡宾(NHC)配体的钌烯烃复分解催化剂,使用计算和实验技术进行了表征,并在许多具有挑战性的反应中进行了测试。预计该催化剂的引发速度比带有异丙基N取代基的类似物快得多。我们还预见到二甲苯侧基在环境温度下的旋转以及溶液中所有四种阻转异构体的存在,这与实验数据一致。这些催化剂在相对较低的温度(45-60°C)和减少的催化剂负载量下,在空间位阻烯烃的各种反应中表现出高活性,包括具有制药意义的复杂多功能底物,例如仰光素前体、菊酸衍生物、大麻素激动剂类似物、α-萜品醇,最后是热不稳定的过氧化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号