当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the factors underlying remyelination arrest by studying the post-transcriptional regulatory mechanisms of cystatin F gene
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-18 , DOI: 10.1111/jnc.15190 Jiayi Li 1, 2, 3 , Wilaiwan Wisessmith Durose 1, 2, 4, 5 , Junko Ito 6 , Akiyoshi Kakita 6 , Yohei Iguchi 3 , Masahisa Katsuno 3 , Kazuo Kunisawa 1, 2, 7 , Takeshi Shimizu 1, 2, 8 , Kazuhiro Ikenaka 1, 2
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-18 , DOI: 10.1111/jnc.15190 Jiayi Li 1, 2, 3 , Wilaiwan Wisessmith Durose 1, 2, 4, 5 , Junko Ito 6 , Akiyoshi Kakita 6 , Yohei Iguchi 3 , Masahisa Katsuno 3 , Kazuo Kunisawa 1, 2, 7 , Takeshi Shimizu 1, 2, 8 , Kazuhiro Ikenaka 1, 2
Affiliation
|
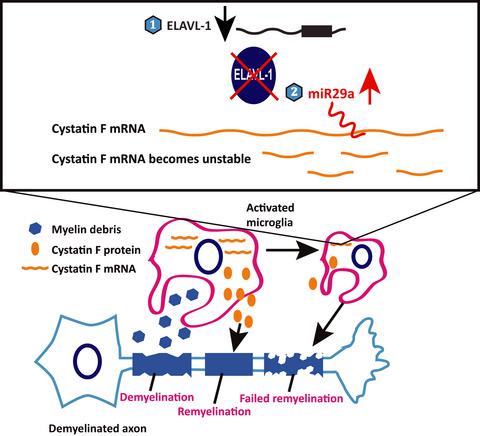
Remyelination plays an important role in determining the fate of demyelinating disorders. However, it is arrested during chronic disease states. Cystatin F, a papain-like lysosomal cysteine proteinase inhibitor, is a crucial regulator of demyelination and remyelination. Using hemizygous proteolipid protein transgenic 4e (PLP4e/-) mice, an animal model of chronic demyelination, we found that cystatin F mRNA expression was induced at 2.5 months of age and up-regulated in the early phase of demyelination, but significantly decreased in the chronic phase. We next investigated cystatin F regulatory factors as potential mechanisms of remyelination arrest in chronic demyelinating disorders. We used the CysF-STOP-tetO::Iba-mtTA mouse model, in which cystatin F gene expression is driven by the tetracycline operator. Interestingly, we found that forced cystatin F mRNA over-expression was eventually decreased. Our findings show that cystatin F expression is modulated post-transcriptionally. We next identified embryonic lethal, abnormal vision, drosophila like RNA-binding protein 1 (ELAVL-1), and miR29a as cystatin F mRNA stabilizing and destabilizing factors, respectively. These roles were confirmed in vitro in NIH3T3 cells. Using postmortem plaque samples from human multiple sclerosis patients, we also confirmed that ELAVL-1 expression was highly correlated with the previously reported expression pattern of cystatin F. These data indicate the important roles of ELAVL-1 and miR29a in regulating cystatin F expression. Furthermore, they provide new insights into potential therapeutic targets for demyelinating disorders.
中文翻译:

通过研究胱抑素F基因的转录后调控机制探索髓鞘再生停滞的相关因素
髓鞘再生在决定脱髓鞘疾病的命运方面起着重要作用。然而,它在慢性疾病状态下被阻止。Cystatin F 是一种木瓜蛋白酶样溶酶体半胱氨酸蛋白酶抑制剂,是脱髓鞘和髓鞘再生的关键调节剂。使用半合子蛋白脂蛋白转基因 4e ( PLP 4e/-) 小鼠,一种慢性脱髓鞘的动物模型,我们发现胱抑素 F mRNA 表达在 2.5 月龄时被诱导,并在脱髓鞘的早期上调,但在慢性期显着降低。我们接下来研究了胱抑素 F 调节因子作为慢性脱髓鞘疾病中髓鞘再生停滞的潜在机制。我们使用了 CysF-STOP-tetO::Iba-mtTA 小鼠模型,其中胱抑素 F 基因表达由四环素操作符驱动。有趣的是,我们发现强制胱抑素 F mRNA 过度表达最终会降低。我们的研究结果表明,胱抑素 F 表达在转录后受到调节。我们接下来将胚胎致死、视力异常、果蝇样 RNA 结合蛋白 1 (ELAVL-1) 和 miR29a 分别鉴定为胱抑素 F mRNA 稳定和不稳定因子。这些作用在体外 NIH3T3 细胞中得到证实。使用来自人类多发性硬化症患者的死后斑块样本,我们还证实 ELAVL-1 表达与先前报道的胱抑素 F 表达模式高度相关。这些数据表明 ELAVL-1 和 miR29a 在调节胱抑素 F 表达中的重要作用。此外,它们为脱髓鞘疾病的潜在治疗目标提供了新的见解。
更新日期:2020-09-18
中文翻译:

通过研究胱抑素F基因的转录后调控机制探索髓鞘再生停滞的相关因素
髓鞘再生在决定脱髓鞘疾病的命运方面起着重要作用。然而,它在慢性疾病状态下被阻止。Cystatin F 是一种木瓜蛋白酶样溶酶体半胱氨酸蛋白酶抑制剂,是脱髓鞘和髓鞘再生的关键调节剂。使用半合子蛋白脂蛋白转基因 4e ( PLP 4e/-) 小鼠,一种慢性脱髓鞘的动物模型,我们发现胱抑素 F mRNA 表达在 2.5 月龄时被诱导,并在脱髓鞘的早期上调,但在慢性期显着降低。我们接下来研究了胱抑素 F 调节因子作为慢性脱髓鞘疾病中髓鞘再生停滞的潜在机制。我们使用了 CysF-STOP-tetO::Iba-mtTA 小鼠模型,其中胱抑素 F 基因表达由四环素操作符驱动。有趣的是,我们发现强制胱抑素 F mRNA 过度表达最终会降低。我们的研究结果表明,胱抑素 F 表达在转录后受到调节。我们接下来将胚胎致死、视力异常、果蝇样 RNA 结合蛋白 1 (ELAVL-1) 和 miR29a 分别鉴定为胱抑素 F mRNA 稳定和不稳定因子。这些作用在体外 NIH3T3 细胞中得到证实。使用来自人类多发性硬化症患者的死后斑块样本,我们还证实 ELAVL-1 表达与先前报道的胱抑素 F 表达模式高度相关。这些数据表明 ELAVL-1 和 miR29a 在调节胱抑素 F 表达中的重要作用。此外,它们为脱髓鞘疾病的潜在治疗目标提供了新的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号