当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Enantioselective [3+2] Cycloaddition of Azomethine Ylides with 2,4‐Dienals: Construction of Remote Stereogenic Centers via 1,6‐Addition Reaction
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/adsc.202000813 Hui Jiang 1 , Leijie Zhou 1 , Biming Mao 2 , Chunhao Yuan 1 , Wei Wang 1 , Yongjun Wu 1 , Cheng Zhang 1 , Hongchao GUO 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/adsc.202000813 Hui Jiang 1 , Leijie Zhou 1 , Biming Mao 2 , Chunhao Yuan 1 , Wei Wang 1 , Yongjun Wu 1 , Cheng Zhang 1 , Hongchao GUO 1
Affiliation
|
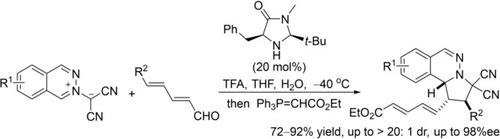
A regio‐ and stereoselective [3+2] cycloaddition of phthalazinium dicyanomethanides with 2,4‐dienals was achieved with the use of a commercially available MacMillan's calalyst via LUMO‐lowering activation. The reaction proceeds through 1,6‐additon to linear 2,4‐dienals with remote control over stereochemistry, affording the cycloadducts having three stereogenic centers.
中文翻译:

带有2,4-二烯醛的甲亚胺叶立德的有机催化对映选择性[3 + 2]环加成反应:通过1,6-加成反应构建远程立体中心
使用市售的MacMillan催化剂通过降低LUMO的活化作用,可以实现邻苯二甲酸和立体选择性的[3 + 2]邻苯二甲腈二氰胺与2,4二环的加成反应。反应通过1,6-己二醛进行,直至线性2,4-二烯醛,并可通过立体化学进行远程控制,从而得到具有三个立体异构中心的环加合物。
更新日期:2020-09-18
中文翻译:

带有2,4-二烯醛的甲亚胺叶立德的有机催化对映选择性[3 + 2]环加成反应:通过1,6-加成反应构建远程立体中心
使用市售的MacMillan催化剂通过降低LUMO的活化作用,可以实现邻苯二甲酸和立体选择性的[3 + 2]邻苯二甲腈二氰胺与2,4二环的加成反应。反应通过1,6-己二醛进行,直至线性2,4-二烯醛,并可通过立体化学进行远程控制,从而得到具有三个立体异构中心的环加合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号