当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 18 F-radiolabeled organophosphine fluorides for thiol-chemoselective peptide conjugation.
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-09-19 , DOI: 10.1002/jlcr.3882 Huanglan Zhuang 1 , Zhide Guo 1 , Rongqiang Zhuang 1 , Xianzhong Zhang 2
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-09-19 , DOI: 10.1002/jlcr.3882 Huanglan Zhuang 1 , Zhide Guo 1 , Rongqiang Zhuang 1 , Xianzhong Zhang 2
Affiliation
|
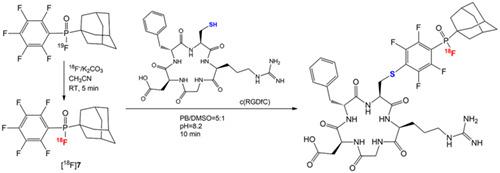
Two 18F‐radiolabeled organofluorophosphine fluorides ([18F]4 and [18F]7) for chemoselective thiol‐conjugation were designed and synthesized via 18F–19F isotopic exchange reaction. This simple and rapid radiofluorination produced both 18F‐radiolabeled fluorides in excellent radiochemical yields (>94%) and radiochemical purity. The optimal reaction conditions are 0.05‐mg substrate, 0.69 mg of potassium carbonate, and dried [18F]F− were mixed in 100‐μl anhydrous acetonitrile at room temperature for 5 min. Both of [18F]4 and [18F]7 showed specificity for thiol‐conjugation with cysteine and have been used in the radiosynthesis of c (RGDfC). The [18F]7 with an adamantanyl‐hindered substituent displayed superior in vitro and in vivo stability.
中文翻译:

用于硫醇-化学选择性肽缀合的 18 F-放射性标记有机膦氟化物的合成。
通过18 F- 19 F 同位素交换反应设计并合成了两种用于化学选择性硫醇缀合的18 F放射性标记有机氟氟化膦([ 18 F] 4和 [ 18 F] 7 )。这种简单而快速的放射性氟化反应产生了18 F 放射性标记的氟化物,具有优异的放射化学收率 (>94%) 和放射化学纯度。最佳反应条件为0.05 mg底物、0.69 mg碳酸钾和干燥的[ 18 F]F -在100 μl无水乙腈中室温混合5 min。 [ 18 F] 4和 [ 18 F] 7均表现出硫醇与半胱氨酸缀合的特异性,并已用于 c (RGDfC) 的放射合成。具有金刚烷基位阻取代基的[ 18 F] 7显示出优异的体外和体内稳定性。
更新日期:2020-09-19
中文翻译:

用于硫醇-化学选择性肽缀合的 18 F-放射性标记有机膦氟化物的合成。
通过18 F- 19 F 同位素交换反应设计并合成了两种用于化学选择性硫醇缀合的18 F放射性标记有机氟氟化膦([ 18 F] 4和 [ 18 F] 7 )。这种简单而快速的放射性氟化反应产生了18 F 放射性标记的氟化物,具有优异的放射化学收率 (>94%) 和放射化学纯度。最佳反应条件为0.05 mg底物、0.69 mg碳酸钾和干燥的[ 18 F]F -在100 μl无水乙腈中室温混合5 min。 [ 18 F] 4和 [ 18 F] 7均表现出硫醇与半胱氨酸缀合的特异性,并已用于 c (RGDfC) 的放射合成。具有金刚烷基位阻取代基的[ 18 F] 7显示出优异的体外和体内稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号