当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overexpression of alpha‐1 antitrypsin in mesenchymal stromal cells improves their intrinsic biological properties and therapeutic effects in nonobese diabetic mice
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/sctm.20-0122 Lili Song 1 , Wenyu Gou 1 , Jingjing Wang 1 , Hua Wei 1 , Jennifer Lee 2 , Charlie Strange 3 , Hongjun Wang 1, 4
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/sctm.20-0122 Lili Song 1 , Wenyu Gou 1 , Jingjing Wang 1 , Hua Wei 1 , Jennifer Lee 2 , Charlie Strange 3 , Hongjun Wang 1, 4
Affiliation
|
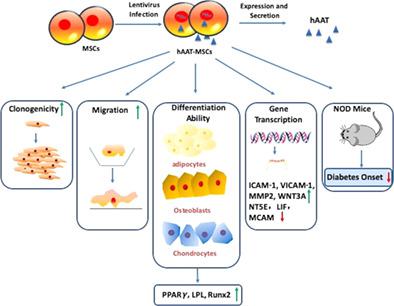
Islet/β cell dysfunction and death caused by autoimmune‐mediated injuries are major features of type 1 diabetes (T1D). Mesenchymal stromal cells (MSCs) have been used for the treatment of T1D in animal models and clinical trials. Based on the anti‐inflammatory effects of alpha‐1 antitrypsin (AAT), we generated human AAT engineered MSCs (hAAT‐MSCs) by infecting human bone marrow‐derived MSCs with the pHAGE CMV‐a1aT‐UBC‐GFP‐W lentiviral vector. We compared the colony forming, differentiation, and migration capacity of empty virus‐treated MSCs (hMSC) and hAAT‐MSCs and tested their protective effects in the prevention of onset of T1D in nonobese diabetic (NOD) mice. hAAT‐MSCs showed increased self‐renewal, better migration and multilineage differentiation abilities compared to hMSCs. In addition, polymerase chain reaction array for 84 MSC‐related genes showed that 23 genes were upregulated, and 3 genes were downregulated in hAAT‐MSCs compared to hMSCs. Upregulated genes include those critical for the stemness (ie, Wnt family member 3A [WNT3A], kinase insert domain receptor [KDR]), migration (intercellular adhesion molecule 1 [ICAM‐1], vascular cell adhesion protein 1 [VICAM‐1], matrix metalloproteinase‐2 [MMP2]), and survival (insulin‐like growth factor 1 [IGF‐1]) of MSCs. Pathway analysis showed that changed genes were related to growth factor activity, positive regulation of cell migration, and positive regulation of transcription. In vivo, a single intravenous infusion of hAAT‐MSCs significantly limited inflammatory infiltration into islets and delayed diabetes onset in the NOD mice compared with those receiving vehicle or hMSCs. Taken together, overexpression of hAAT in MSCs improved intrinsic biological properties of MSCs needed for cellular therapy for the treatment of T1D.
中文翻译:

间充质基质细胞中α-1抗胰蛋白酶的过度表达改善了非肥胖糖尿病小鼠的内在生物学特性和治疗效果
自身免疫介导的损伤引起的胰岛/β细胞功能障碍和死亡是 1 型糖尿病 (T1D) 的主要特征。间充质基质细胞 (MSC) 已在动物模型和临床试验中用于治疗 T1D。基于α-1抗胰蛋白酶(AAT)的抗炎作用,我们通过用pHAGE CMV-a1aT-UBC-GFP-W慢病毒载体感染人骨髓源性MSC来产生人AAT工程MSC(hAAT-MSC)。我们比较了空病毒处理的 MSC (hMSC) 和 hAAT-MSC 的集落形成、分化和迁移能力,并测试了它们在预防非肥胖糖尿病 (NOD) 小鼠中预防 T1D 发病的保护作用。与 hMSC 相比,hAAT-MSC 表现出更强的自我更新、更好的迁移和多向分化能力。此外,84个MSC相关基因的聚合酶链反应芯片显示,与hMSC相比,hAAT-MSC中23个基因上调,3个基因下调。上调的基因包括对干性至关重要的基因(即 Wnt 家族成员 3A [WNT3A]、激酶插入结构域受体 [KDR])、迁移(细胞间粘附分子 1 [ICAM-1]、血管细胞粘附蛋白 1 [VICAM-1] 、基质金属蛋白酶-2 [MMP2])和 MSC 的存活(胰岛素样生长因子 1 [IGF-1])。通路分析表明,变化的基因与生长因子活性、细胞迁移的正向调节和转录的正向调节有关。在体内,与接受载体或 hMSC 的小鼠相比,单次静脉输注 hAAT-MSC 显着限制了 NOD 小鼠胰岛的炎症浸润,并延迟了糖尿病的发病。 综上所述,hAAT 在 MSC 中的过度表达改善了 MSC 的内在生物学特性,这是治疗 T1D 的细胞疗法所需的。
更新日期:2020-09-18
中文翻译:

间充质基质细胞中α-1抗胰蛋白酶的过度表达改善了非肥胖糖尿病小鼠的内在生物学特性和治疗效果
自身免疫介导的损伤引起的胰岛/β细胞功能障碍和死亡是 1 型糖尿病 (T1D) 的主要特征。间充质基质细胞 (MSC) 已在动物模型和临床试验中用于治疗 T1D。基于α-1抗胰蛋白酶(AAT)的抗炎作用,我们通过用pHAGE CMV-a1aT-UBC-GFP-W慢病毒载体感染人骨髓源性MSC来产生人AAT工程MSC(hAAT-MSC)。我们比较了空病毒处理的 MSC (hMSC) 和 hAAT-MSC 的集落形成、分化和迁移能力,并测试了它们在预防非肥胖糖尿病 (NOD) 小鼠中预防 T1D 发病的保护作用。与 hMSC 相比,hAAT-MSC 表现出更强的自我更新、更好的迁移和多向分化能力。此外,84个MSC相关基因的聚合酶链反应芯片显示,与hMSC相比,hAAT-MSC中23个基因上调,3个基因下调。上调的基因包括对干性至关重要的基因(即 Wnt 家族成员 3A [WNT3A]、激酶插入结构域受体 [KDR])、迁移(细胞间粘附分子 1 [ICAM-1]、血管细胞粘附蛋白 1 [VICAM-1] 、基质金属蛋白酶-2 [MMP2])和 MSC 的存活(胰岛素样生长因子 1 [IGF-1])。通路分析表明,变化的基因与生长因子活性、细胞迁移的正向调节和转录的正向调节有关。在体内,与接受载体或 hMSC 的小鼠相比,单次静脉输注 hAAT-MSC 显着限制了 NOD 小鼠胰岛的炎症浸润,并延迟了糖尿病的发病。 综上所述,hAAT 在 MSC 中的过度表达改善了 MSC 的内在生物学特性,这是治疗 T1D 的细胞疗法所需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号