当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells‐based therapy product
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/sctm.20-0242 Balendu Shekhar Jha 1 , Mitra Farnoodian 2 , Kapil Bharti 2
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-09-18 , DOI: 10.1002/sctm.20-0242 Balendu Shekhar Jha 1 , Mitra Farnoodian 2 , Kapil Bharti 2
Affiliation
|
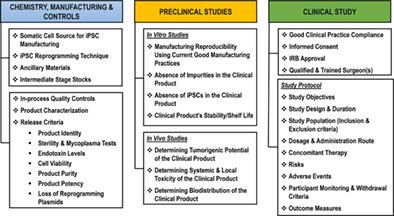
Induced pluripotent stem cells (iPSC)‐based therapies have been hailed as the future of regenerative medicine because of their potential to provide treatment options for most degenerative diseases. A key promise of iPSC‐based therapies is the possibility of an autologous transplant that may engraft better in the longer‐term due to its compatibility with the patient's immune system. Despite over a decade of research, clinical translation of autologous iPSC‐based therapies has been slow—partly due to a lacking pre‐defined regulatory path. Here, we outline regulatory considerations for developing an autologous iPSC‐based product and challenges associated with the clinical manufacturing of autologous iPSCs and their derivatives. These challenges include donor tissue source, reprogramming methods, heterogeneity of differentiated cells, controls for the manufacturing process, and preclinical considerations. A robust manufacturing process with appropriate quality controls and well‐informed, prospectively designed preclinical studies provide a path toward successful approval of autologous iPSC‐based therapies.
中文翻译:

开发基于自体诱导多能干细胞的治疗产品的 I 期研究新药应用的监管考虑因素
基于诱导多能干细胞(iPSC)的疗法被誉为再生医学的未来,因为它们有可能为大多数退行性疾病提供治疗选择。基于 iPSC 的疗法的一个关键承诺是自体移植的可能性,由于其与患者免疫系统的相容性,从长远来看,这种移植可能会更好地植入。尽管进行了十多年的研究,基于自体 iPSC 的疗法的临床转化进展缓慢,部分原因是缺乏预先定义的监管路径。在这里,我们概述了开发基于自体 iPSC 的产品的监管考虑因素以及与自体 iPSC 及其衍生物的临床制造相关的挑战。这些挑战包括供体组织来源、重编程方法、分化细胞的异质性、制造过程的控制以及临床前考虑因素。稳健的制造工艺、适当的质量控制和信息灵通、前瞻性设计的临床前研究为成功批准基于自体 iPSC 的疗法提供了一条途径。
更新日期:2020-09-18
中文翻译:

开发基于自体诱导多能干细胞的治疗产品的 I 期研究新药应用的监管考虑因素
基于诱导多能干细胞(iPSC)的疗法被誉为再生医学的未来,因为它们有可能为大多数退行性疾病提供治疗选择。基于 iPSC 的疗法的一个关键承诺是自体移植的可能性,由于其与患者免疫系统的相容性,从长远来看,这种移植可能会更好地植入。尽管进行了十多年的研究,基于自体 iPSC 的疗法的临床转化进展缓慢,部分原因是缺乏预先定义的监管路径。在这里,我们概述了开发基于自体 iPSC 的产品的监管考虑因素以及与自体 iPSC 及其衍生物的临床制造相关的挑战。这些挑战包括供体组织来源、重编程方法、分化细胞的异质性、制造过程的控制以及临床前考虑因素。稳健的制造工艺、适当的质量控制和信息灵通、前瞻性设计的临床前研究为成功批准基于自体 iPSC 的疗法提供了一条途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号