当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, in vitro cytotoxicity, ADME, and molecular docking studies of benzimidazole‐bearing furanone derivatives
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-09-17 , DOI: 10.1002/jccs.202000130 Asif Husain 1 , Medha Bhutani 1 , Shazia Parveen 2, 3 , Shah Alam Khan 4 , Aftab Ahmad 5 , Md Azhar Iqbal 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-09-17 , DOI: 10.1002/jccs.202000130 Asif Husain 1 , Medha Bhutani 1 , Shazia Parveen 2, 3 , Shah Alam Khan 4 , Aftab Ahmad 5 , Md Azhar Iqbal 1
Affiliation
|
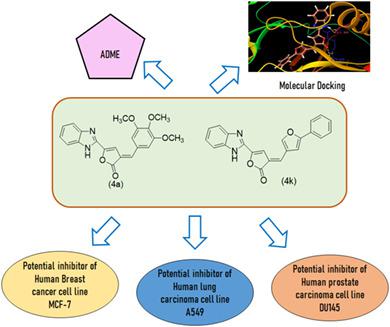
A series of benzimidazole‐derived–furanones (4a–l) were synthesized, characterized, and explored for their in vitro anticancer activities. The pharmacokinetic parameters assessed revealed that all the compounds followed the Lipinski's rule of five, making them potential drug candidates. Further, the results of anticancer activity revealed that (E)‐5‐(1H‐benzo[d]imidazol‐2‐yl)‐3‐(3,4,5‐trimethoxybenzylidene)furan‐2(3H)‐one (4a), was active against A549, MCF7, and DU145 with an IC50 values of 10.4 ± 0.39, 11.1 ± 0.43, and 10.7 ± 0.19 μM, respectively. While another compound (E)‐5‐(1H‐benzo[d]imidazol‐2‐yl)‐3‐([5‐phenyl]furan‐2‐yl)furan‐2(3H)‐one (4k) also exhibited good activity against A549, MCF7, and DU145 with IC50 values of 11.4 ± 0.39, 9.1 ± 0.43, and 12.7 ± 0.19 μM, respectively. Doxorubicin was used as the standard drug. Further, molecular docking studies were carried out to provide binding mode into the binding sites of vascular endothelial growth factor receptor (VEGFR). Docking scores and binding energies corroborated well with the results of experimental anticancer activity. Pharmacokinetic (ADME) parameters of the potent derivatives were also found to be in an acceptable range. The benzimidazole‐furanonone conjugates seem to be a potential source for the further development of potent cytotoxic agents.
中文翻译:

含苯并咪唑的呋喃酮衍生物的合成,体外细胞毒性,ADME和分子对接研究
合成,表征并研究了一系列苯并咪唑衍生的呋喃酮(4a - l)的体外抗癌活性。评估的药代动力学参数表明,所有化合物均遵循Lipinski的5条规则,使其成为潜在的候选药物。此外,抗癌活性的结果表明,(E)-5(1 H-苯并[ d ]咪唑-2-基)-3-(3,4,5-三甲氧基亚苄基)呋喃-2(3 H)- (4a)对A549,MCF7和DU145有活性,IC 50值分别为10.4±0.39、11.1±0.43和10.7±0.19μM。而另一种化合物(E)‐5‐(1 H-苯并[ d ]咪唑-2-基)-3-([[5-苯基]呋喃-2-基)呋喃-2(3 H)-one(4k)还对A549,MCF7和DU145表现出良好的活性IC 50分别为11.4±0.39、9.1±0.43和12.7±0.19μM。阿霉素被用作标准药物。此外,进行了分子对接研究以提供与血管内皮生长因子受体(VEGFR)的结合位点的结合模式。对接分数和结合能与实验抗癌活性的结果得到了很好的证实。还发现有效衍生物的药代动力学(ADME)参数在可接受的范围内。苯并咪唑-呋喃酮偶联物似乎是进一步开发有效细胞毒性剂的潜在来源。
更新日期:2020-09-17
中文翻译:

含苯并咪唑的呋喃酮衍生物的合成,体外细胞毒性,ADME和分子对接研究
合成,表征并研究了一系列苯并咪唑衍生的呋喃酮(4a - l)的体外抗癌活性。评估的药代动力学参数表明,所有化合物均遵循Lipinski的5条规则,使其成为潜在的候选药物。此外,抗癌活性的结果表明,(E)-5(1 H-苯并[ d ]咪唑-2-基)-3-(3,4,5-三甲氧基亚苄基)呋喃-2(3 H)- (4a)对A549,MCF7和DU145有活性,IC 50值分别为10.4±0.39、11.1±0.43和10.7±0.19μM。而另一种化合物(E)‐5‐(1 H-苯并[ d ]咪唑-2-基)-3-([[5-苯基]呋喃-2-基)呋喃-2(3 H)-one(4k)还对A549,MCF7和DU145表现出良好的活性IC 50分别为11.4±0.39、9.1±0.43和12.7±0.19μM。阿霉素被用作标准药物。此外,进行了分子对接研究以提供与血管内皮生长因子受体(VEGFR)的结合位点的结合模式。对接分数和结合能与实验抗癌活性的结果得到了很好的证实。还发现有效衍生物的药代动力学(ADME)参数在可接受的范围内。苯并咪唑-呋喃酮偶联物似乎是进一步开发有效细胞毒性剂的潜在来源。









































 京公网安备 11010802027423号
京公网安备 11010802027423号