当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Enantioconvergent Cross-Coupling of Racemic Alkyl Bromides with Azole C(sp2)-H Bonds.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-19 , DOI: 10.1002/anie.202009527 Xiao-Long Su 1, 2 , Liu Ye 3 , Ji-Jun Chen 2 , Xiao-Dong Liu 2 , Sheng-Peng Jiang 2 , Fu-Li Wang 2 , Lin Liu 2 , Chang-Jiang Yang 2 , Xiao-Yong Chang 2 , Zhong-Liang Li 3 , Qiang-Shuai Gu 3 , Xin-Yuan Liu 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-19 , DOI: 10.1002/anie.202009527 Xiao-Long Su 1, 2 , Liu Ye 3 , Ji-Jun Chen 2 , Xiao-Dong Liu 2 , Sheng-Peng Jiang 2 , Fu-Li Wang 2 , Lin Liu 2 , Chang-Jiang Yang 2 , Xiao-Yong Chang 2 , Zhong-Liang Li 3 , Qiang-Shuai Gu 3 , Xin-Yuan Liu 2
Affiliation
|
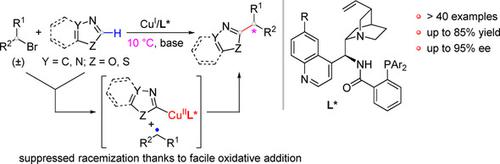
The development of enantioconvergent cross‐coupling of racemic alkyl halides directly with heteroarene C(sp2)−H bonds has been impeded by the use of a base at elevated temperature that leads to racemization. We herein report a copper(I)/cinchona‐alkaloid‐derived N,N,P‐ligand catalytic system that enables oxidative addition with racemic alkyl bromides under mild conditions. Thus, coupling with azole C(sp2)−H bonds has been achieved in high enantioselectivity, affording a number of potentially useful α‐chiral alkylated azoles, such as 1,3,4‐oxadiazoles, oxazoles, and benzo[d]oxazoles as well as 1,3,4‐triazoles, for drug discovery. Mechanistic experiments indicated facile deprotonation of an azole C(sp2)−H bond and the involvement of alkyl radical species under the reaction conditions.
中文翻译:

铜催化的外消旋烷基溴与对映体C(sp2)-H键的铜对映体交叉偶联。
外消旋烷基卤化物与杂芳烃C(sp 2)-H键直接对映对合交叉偶联的发展已被在高温下使用导致外消旋作用的碱所阻碍。我们在此报告了铜(I)/金鸡纳生物碱衍生的N,N,P配体催化体系,该体系能够在温和条件下与外消旋烷基溴化物进行氧化加成。因此,已经以高对映选择性实现了与吡咯C(sp 2)-H键的偶联,从而提供了许多潜在有用的α-手性烷基化吡咯,例如1,3,4-恶二唑,恶唑和苯并[ d]]恶唑以及1,3,4-三唑,用于药物发现。机理实验表明,在反应条件下,吡咯C(sp 2)-H键容易去质子化,并且涉及烷基自由基。
更新日期:2020-09-19
中文翻译:

铜催化的外消旋烷基溴与对映体C(sp2)-H键的铜对映体交叉偶联。
外消旋烷基卤化物与杂芳烃C(sp 2)-H键直接对映对合交叉偶联的发展已被在高温下使用导致外消旋作用的碱所阻碍。我们在此报告了铜(I)/金鸡纳生物碱衍生的N,N,P配体催化体系,该体系能够在温和条件下与外消旋烷基溴化物进行氧化加成。因此,已经以高对映选择性实现了与吡咯C(sp 2)-H键的偶联,从而提供了许多潜在有用的α-手性烷基化吡咯,例如1,3,4-恶二唑,恶唑和苯并[ d]]恶唑以及1,3,4-三唑,用于药物发现。机理实验表明,在反应条件下,吡咯C(sp 2)-H键容易去质子化,并且涉及烷基自由基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号