当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Formosalides: Structure Determination by Total Synthesis.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-18 , DOI: 10.1002/anie.202011472 Saskia Schulthoff 1 , James Y Hamilton 1 , Marc Heinrich 1 , Yonghoon Kwon 1 , Conny Wirtz 1 , Alois Fürstner 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-09-18 , DOI: 10.1002/anie.202011472 Saskia Schulthoff 1 , James Y Hamilton 1 , Marc Heinrich 1 , Yonghoon Kwon 1 , Conny Wirtz 1 , Alois Fürstner 1
Affiliation
|
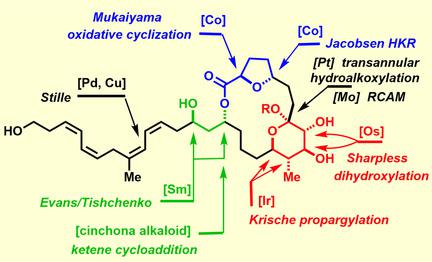
Total synthesis allowed the constitution of the cytotoxic marine macrolides of the formosalide family to be confirmed and their previously unknown stereostructure to be assigned with confidence. The underlying blueprint was inherently modular to ensure that each conceivable isomer could be reached. This flexibility derived from the use of strictly catalyst controlled transformations to set the stereocenters, except for the anomeric position, which is under thermodynamic control; as an extra safety measure, all stereogenic centers were set prior to ring closure to preclude any interference of the conformation adopted by the macrolactone rings of the different diastereomers. Late‐stage macrocyclization by ring‐closing alkyne metathesis was followed by a platinum‐catalyzed transannular 6‐exo‐dig hydroalkoxylation/ketalization to craft the polycyclic frame. The side chain featuring a very labile unsaturation pattern was finally attached to the core by Stille coupling.
中文翻译:

福尔莫内酯:全合成结构测定。
全合成使得福尔莫内酯家族的细胞毒性海洋大环内酯的构成得以确认,并且可以放心地确定其先前未知的立体结构。底层蓝图本质上是模块化的,以确保可以实现每种可想象的异构体。这种灵活性源自使用严格的催化剂控制的转化来设置立构中心,但异头位置除外,该位置受热力学控制;作为一项额外的安全措施,所有立体中心均在环闭合之前设定,以排除不同非对映异构体的大内酯环所采用的构象的任何干扰。通过闭环炔复分解进行后期大环化,然后进行铂催化的跨环 6-exo-dig 加氢烷氧基化/缩酮化,以形成多环框架。具有非常不稳定的不饱和模式的侧链最终通过 Stille 耦合连接到核心上。
更新日期:2020-09-18
中文翻译:

福尔莫内酯:全合成结构测定。
全合成使得福尔莫内酯家族的细胞毒性海洋大环内酯的构成得以确认,并且可以放心地确定其先前未知的立体结构。底层蓝图本质上是模块化的,以确保可以实现每种可想象的异构体。这种灵活性源自使用严格的催化剂控制的转化来设置立构中心,但异头位置除外,该位置受热力学控制;作为一项额外的安全措施,所有立体中心均在环闭合之前设定,以排除不同非对映异构体的大内酯环所采用的构象的任何干扰。通过闭环炔复分解进行后期大环化,然后进行铂催化的跨环 6-exo-dig 加氢烷氧基化/缩酮化,以形成多环框架。具有非常不稳定的不饱和模式的侧链最终通过 Stille 耦合连接到核心上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号