Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2020-09-20 , DOI: 10.1016/j.jece.2020.104498 Hanane Afanga , Hicham Zazou , Fatima Ezzahra Titchou , Jamila El Gaayda , Flamur Sopaj , Rachid Ait Akbour , Mohamed Hamdani
|
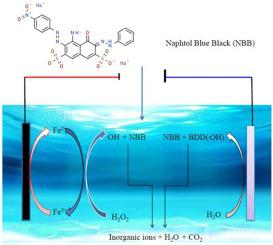
The electrochemical oxidation of Naphthol Blue Black (NBB) solution by means of anodic oxidation with electro-generated H2O2 (AO-H2O2) and Electro-Fenton (EF) was studied, using boron doped diamond (BDD)/ carbon felt (CF) cell. The experiments were carried out in NaCl and Na2SO4 as supporting electrolytes with initial concentration of 0.1 mM of NBB. The studied parameters were pH, applied current, concentration of Fenton catalyst, concentration of supporting electrolytes, and Cl-/SO42- mixture. The degradation of NBB was almost total when NaCl was used compared to Na2SO4, thanks to the electro-generated active chlorine (HClO/ClO-). The higher degradation is found with EF compared to AO-H2O2 process, the kinetic of degradation of NBB always follows a pseudo first-order reaction. The optimum conditions for the mineralization of NBB (i.e., 0.1 mM NBB, 50 mM Na2SO4 at pH 3.0, 0.1 mM Fe2+, and a current of 300 mA) were determined. These conditions yielded a total color removal in less than 10 min and 98% of total organic carbon (TOC) removal at 120 min electrolysis time. The biochemical oxygen demand/ Chemical oxygen demand (BOD/COD) ratio was decreased from 0.5 to 0.3, during the same timescales. Whereas, the mineralization current efficiency (MCE%) dropped from 21.5% to 0.05% in the electrolysis time range from 15- 120 min suggesting the concomitant parasitic reactions. The evolution of nitrite NO2-, nitrate NO3-, ammonium NH4+, and sulfate SO42- concentrations were also followed as the end-products during the electrolysis.
中文翻译:

使用BDD /碳毡电池用不同的辅助电解质对萘酚蓝黑进行电化学氧化
通过用阳极氧化的装置的萘酚蓝黑(NBB)的溶液的电化学氧化电产生ħ 2 ö 2(AO-H 2 ö 2)和电芬顿(EF)进行了研究,使用硼掺杂金刚石(BDD)/碳毡(CF)电池。实验在NaCl和Na 2 SO 4作为支持电解质中进行,初始浓度为0.1 mM NBB。研究的参数为pH,施加电流,Fenton催化剂的浓度,支持电解质的浓度和Cl- / SO 4 2-混合物。与Na 2 SO 4相比,使用NaCl时NBB的降解几乎完全消失。,这要归功于电产生的活性氯(HClO / ClO-)。与AO-H 2 O 2工艺相比,EF的降解更高,NBB的降解动力学始终遵循伪一级反应。对于NBB(即,0.1mM的NBB,50mM的Na组成的矿化的最佳条件2 SO 4在pH 3.0,0.1mM的铁2+,并确定300 mA的电流。这些条件可在不到10分钟的时间内实现全部脱色,在120分钟的电解时间下可去除98%的有机碳。在相同的时间范围内,生化需氧量/化学需氧量(BOD / COD)比从0.5降低至0.3。而在15至120分钟的电解时间范围内,矿化电流效率(MCE%)从21.5%降至0.05%,表明伴随有寄生反应。电解过程中,最终产物亚硝酸盐NO 2-,硝酸盐NO 3-,铵NH 4 +和硫酸盐SO 4 2-的浓度也随之变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号