Water Research ( IF 11.4 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.watres.2020.116424 Juan Li , Jin Jiang , Tarek Manasfi , Urs von Gunten
|
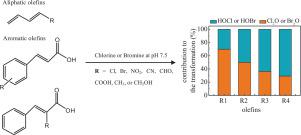
Hypochlorous acid (HOCl) is typically assumed to be the primary reactive species in free available chlorine (FAC) solutions. Lately, it has been shown that less abundant chlorine species such as chlorine monoxide (Cl2O) and chlorine (Cl2) can also influence the kinetics of the abatement of certain compounds during chlorination. In this study, the chlorination as well as bromination kinetics and mechanisms of 12 selected olefins (including 3 aliphatic and 9 aromatic olefins) with different structures were explored. HOCl shows a low reactivity towards selected olefins with species-specific second-order rate constants <1.0 M−1s−1, about 4-6 orders of magnitude lower than those of Cl2O and Cl2. HOCl is the dominant chlorine species during chlorination of olefins under typical drinking water conditions, while Cl2O and Cl2 are likely to play important roles at high FAC concentration near circum-neutral pH (for Cl2O) or at high Cl− concentration under acidic conditions (for Cl2). Bromination of the 12 olefins suggests that HOBr and Br2O are the major reactive species at pH 7.5 with species-specific second-order rate constants of Br2O nearly 3-4 orders of magnitude higher than of HOBr (ranging from <0.01 to >103 M−1s−1). The reactivities of chlorine and bromine species towards olefins follow the order of HOCl < HOBr < Br2O < Cl2O ≈ Cl2. Generally, electron-donating groups (e.g., CH2OH- and CH3-) enhances the reactivities of olefins towards chlorine and bromine species by a factor of 3-102, while electron-withdrawing groups (e.g., Cl-, Br-, NO2-, COOH-, CHO-, -COOR, and CN-) reduce the reactivities by a factor of 3-104. A reasonable linear free energy relationship (LFER) between the species-specific second-order rate constants of Br2O or Cl2O reactions with aromatic olefins and their Hammett σ + was established with a more negative ρ value for Br2O than for Cl2O, indicating that Br2O is more sensitive to substitution effects. Chlorinated products including HOCl-adducts and decarboxylated Cl-adduct were identified during chlorination of cinnamic acid by high-performance liquid chromatography/high resolution mass spectrometry (HPLC/HRMS).
中文翻译:

烯烃的氯化和溴化:动力学和机理
次氯酸(HOCl)通常被认为是游离氯(FAC)溶液中的主要反应物种。最近,已经显示出较少的丰富的氯物种,例如一氧化氯(Cl 2 O)和氯(Cl 2),也会影响氯化过程中某些化合物的减排动力学。在这项研究中,探讨了十二种选择的具有不同结构的烯烃(包括3个脂族和9个芳族烯烃)的氯化以及溴化动力学和机理。HOCl对特定烯烃的物种特异性二阶速率常数<1.0 M -1 s -1,比Cl 2 O和Cl 2低约4-6个数量级,显示出对所选烯烃的低反应性。次氯酸是主要的氯种典型的饮用水条件下,烯烃的氯化过程中,而氯2 O和氯2很可能在不久的circum中性pH值高FAC浓度扮演重要角色(氯2 O)或高氯-浓度在酸性条件下(对于Cl 2)。12个烯烃的溴化表明,次溴酸和Br 2的O在含有溴的物种特异性的二阶速率常数pH为7.5的主要反应性物质2 ö将近3-4个数量级比次溴酸的更高(从<0.01至> 10 3 M -1 s -1)。氯和溴物种朝向烯烃反应性遵循次氯酸<次溴酸<BR的顺序2 0 <氯2 ö≈氯2。通常,供电子基团(例如,CH 2 OH-和CH 3-)将烯烃对氯和溴物种的反应性提高3-10 2倍,而吸电子基团(例如,Cl-,Br- ,NO 2-,COOH-,CHO-,-COOR和CN-)降低反应活性3-10 4倍。Br 2 O或Cl 2 O与芳族烯烃及其哈米特反应的物种特异性二阶速率常数之间的合理线性自由能关系(LFER) 建立的σ +对Br 2 O的负ρ值大于对Cl 2 O的ρ值,表明Br 2 O对取代效应更敏感。通过高效液相色谱/高分辨率质谱(HPLC / HRMS)在肉桂酸氯化过程中鉴定出包括HOCl加合物和脱羧Cl加合物在内的氯化产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号