Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.tetlet.2020.152465 Gabriel J. Pongdee , Kathryn G. Bell , Peri R. Prestwood , Rongson Pongdee
|
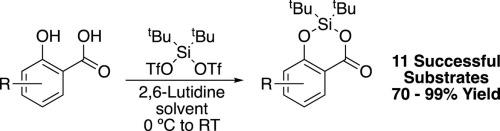
The use of di-tert-butylsilyl bis(trifluoromethanesulfonate) has been used to form cyclic protecting groups for substituted salicylic acids. The reaction works well in the presence of a variety of electron-donating groups (EDG) affording the protected compounds in moderate to excellent yields (70–99%) in most cases. In addition, a handful of electron-withdrawing groups (EWG) also provided the corresponding protected silylene derivatives in good yields (77–83%). However, substrates bearing additional ortho -substitution of the carboxylic acid moiety as well as strongly deactivating groups on the aromatic ring did not undergo reaction.
中文翻译:

二-叔-butylsilylene作为用于取代的水杨酸的保护基团
双(三氟甲磺酸)二叔丁基甲硅烷基酯的使用已用于形成取代的水杨酸的环状保护基。在多种给电子基团(EDG)的存在下,该反应效果很好,在大多数情况下,以中等至极好的收率(70-99%)提供受保护的化合物。此外,少数吸电子基团(EWG)还以良好的收率(77–83%)提供了相应的受保护的甲硅烷基衍生物。然而,带有羧酸部分的额外邻位取代以及芳环上的强减活基团的底物没有发生反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号