Protein Expression and Purification ( IF 1.4 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.pep.2020.105761 Yiyi Gong 1 , Meiqi Yi 2 , Lin Zhang 3 , Shan Feng 4 , Haiteng Deng 3
|
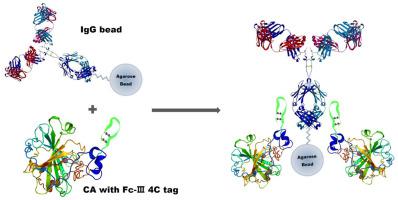
Development of new affinity tags is important for recombinant protein expression and purification. Based on our earlier work, we devised an affinity tag by addition of two cysteine residues onto the N- and C-termini of the Fc-III peptide and designated as the Fc–III–4C tag, in which four cysteine residues form two disulfide linkages. The binding affinity of Fc–III–4C tag to human IgG is measured as 2.28 nM (Kd) and is 100 times higher than that of the Fc-III tag to IgG. Fc–III–4C tagged carbonic anhydrase (CA) can be effectively purified with IgG-immobilized beads, and Fc–III–4C tag does not possess adverse effects on the structure and stability of CA. Furthermore, the Fc–III–4C tagged protein binds to multiple transition metal ions, which enhances activities of enzymes that use metal ions as co-factors. These results suggest that Fc–III–4C tag is a useful tool for expression and purification of recombinant proteins and enhances the activities of some fusion proteins that use Zn2+ or Cu2+ as cofactors.
中文翻译:

通过使用碳酸酐酶作为模型蛋白表征基于Fc-III-4C的重组蛋白表达系统。
开发新的亲和标签对于重组蛋白表达和纯化很重要。根据我们先前的工作,我们通过在Fc-III肽的N和C末端添加两个半胱氨酸残基设计了一种亲和标签,并命名为Fc–III–4C标签,其中四个半胱氨酸残基形成两个二硫键联系。Fc–III–4C标签与人IgG的结合亲和力测量为2.28 nM(K d),比IgG的Fc-III标签高100倍。Fc-III-4C标签的碳酸酐酶(CA)可以用IgG固定的珠子有效地纯化,并且FcIII-4C标签对CA的结构和稳定性没有不利影响。此外,Fc-III-4C标记的蛋白质与多种过渡金属离子结合,从而增强了将金属离子用作辅助因子的酶的活性。这些结果表明,Fc–III–4C标签是表达和纯化重组蛋白的有用工具,并增强了一些使用Zn 2+或Cu 2+作为辅因子的融合蛋白的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号