Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.molmet.2020.101085 Melanie J Mittenbühler 1 , Katarzyna Saedler 1 , Hendrik Nolte 2 , Lara Kern 1 , Jun Zhou 3 , Shu-Bing Qian 3 , Lydia Meder 4 , Roland T Ullrich 5 , Jens C Brüning 6 , F Thomas Wunderlich 6
|
Objective
Single-nucleotide polymorphisms in the FTO gene encoding an m6Am and an m6A demethylase are associated with obesity. Moreover, recent studies have linked a dysregulation of m6A modifications and its machinery, including FTO, to the development of several forms of cancers. However, the functional role of hepatic FTO in metabolism and the development and progression of hepatocellular carcinoma (HCC), a proteotypic obesity-associated cancer, remains unclear. Thus, we aimed to reveal the role of hepatic FTO in metabolism and in the initiation and progression of HCC in vivo.
Methods
We generated mice with hepatic FTO deficiency (FTOL−KO). The effect of hepatic FTO on metabolism was investigated by extensive metabolic phenotyping. To determine the impact of hepatic FTO on HCC development, FTOL−KO and Ctrl mice were subjected to long-term diethylnitrosamine (DEN)-induced HCC-development and the tumor initiation phase was examined via a short-term DEN protocol.
Results
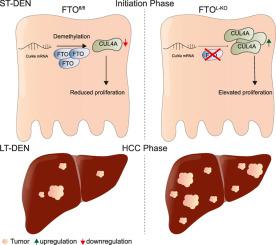
In long-term DEN experiments, FTOL−KO mice exhibit increased HCC burden compared to Ctrl mice. In the tumor initiation phase, Ctrl mice display a dynamic regulation of FTO upon induction of liver damage, while this response is abrogated in FTO-deficient mice. Proteomic analyses revealed that liver damage-induced increases in FTO expression reduce CUL4A protein abundance. Functionally, simultaneous knockdown of Cul4a reverses the increased hepatocyte proliferation observed upon loss of FTO.
Conclusion
Collectively, our study demonstrates that hepatic FTO is dispensable for the control of energy homeostasis and glucose metabolism. However, we show a protective function of FTO in liver carcinogenesis and suggest the FTO-dependent dynamic mRNA demethylation of Cul4a in the initiation of HCC development contributes to this effect.
中文翻译:

肝脏 FTO 对代谢的调节是不必要的,但会抵消体内 HCC 的发展。
客观的
编码 m 6 Am 和 m 6 A 脱甲基酶的 FTO 基因中的单核苷酸多态性与肥胖有关。此外,最近的研究已将 m 6 A 修饰及其机制(包括 FTO)的失调与多种癌症的发展联系起来。然而,肝脏 FTO 在代谢和肝细胞癌 (HCC)(一种蛋白质型肥胖相关癌症)的发展和进展中的功能作用仍不清楚。因此,我们的目的是揭示肝FTO的新陈代谢,并在启动和肝癌的进展中的作用在体内。
方法
我们产生了肝脏 FTO 缺乏症 (FTO L-KO ) 的小鼠。通过广泛的代谢表型研究了肝脏 FTO 对代谢的影响。为了确定肝脏 FTO 对 HCC 发展的影响,FTO L-KO和 Ctrl 小鼠接受长期二乙基亚硝胺 (DEN) 诱导的 HCC 发展,并通过短期 DEN 协议检查肿瘤起始阶段。
结果
在长期 DEN 实验中,与 Ctrl 小鼠相比,FTO L-KO小鼠表现出增加的 HCC 负担。在肿瘤起始阶段,Ctrl 小鼠在诱导肝损伤时表现出对 FTO 的动态调节,而这种反应在 FTO 缺陷小鼠中被取消。蛋白质组学分析表明,肝损伤诱导的 FTO 表达增加会降低 CUL4A 蛋白丰度。在功能上,同时敲低Cul4a 可以逆转 FTO 缺失时观察到的肝细胞增殖增加。
结论
总的来说,我们的研究表明,肝脏 FTO 对于控制能量稳态和葡萄糖代谢是可有可无的。然而,我们显示了 FTO 在肝癌发生中的保护作用,并表明在HCC 发展的起始过程中Cul4a的 FTO 依赖性动态 mRNA 去甲基化有助于这种效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号