Molecular and Biochemical Parasitology ( IF 1.4 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.molbiopara.2020.111319 Blessing O Akumadu 1 , Ramesh Pandian 1 , Jessica Olfsen 1 , Roland Worth 1 , Monare Thulo 1 , Tshireletso Mentor 1 , Sylvia Fanucchi 1 , Yasien Sayed 1 , Heini W Dirr 1 , Ikechukwu Achilonu 1
|
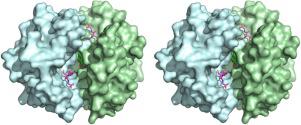
Schistosoma japonicum glutathione transferase (Sj26GST), an enzyme central to detoxification of electrophilic compounds in the parasite, is upregulated in response to drug treatment. Therefore, Sj26GST may serve as a potential therapeutic target for the treatment of schistosomiasis. Herewith, we describe the structural basis of inhibition of Sj26GST by ellagic acid (EA). Using 1-chloro-2,4-dinitrobenzene and reduced glutathione (GSH) as Sj26GST substrates, EA was shown to inhibit Sj26GST activity by 66 % with an IC50 of 2.4 μM. Fluorescence spectroscopy showed that EA altered the polarity of the environment of intrinsic tryptophan and that EA decreased (in a dose-dependent manner) the interaction between Sj26GST and 8-Anilino-1-naphthalenesulfonate (ANS), which is a known GST H-site ligand. Thermodynamic studies indicated that the interaction between Sj26GST and EA is spontaneous (ΔG = -29.88 ± 0.07 kJ/mol), enthalpically-driven (ΔH = -9.48 ± 0.42 kJ/mol) with a favourable entropic change (ΔS = 20.40 ± 0.08 kJ/mol/K), and with a stoichiometry of four EA molecules bound per Sj26GST dimer. The 1.53 Å-resolution Sj26GST crystal structure (P 21 21 21 space group) complexed with GSH and EA shows that EA binds primarily at the dimer interface, stabilised largely by Van der Waal forces and H-bonding. Besides, EA bound near the H-site and less than 3.5 Å from the ε-NH2 of the γ-glutamyl moiety of GSH, in each subunit.
中文翻译:

鞣花酸抑制日本血吸虫谷胱甘肽转移酶的分子基础:对生物物理和结构研究的见解。
日本血吸虫谷胱甘肽转移酶(Sj26GST)是寄生虫中亲电子化合物解毒的核心酶,可响应药物治疗而上调。因此,Sj26GST可以作为血吸虫病的潜在治疗靶标。因此,我们描述了鞣花酸(EA)抑制Sj26GST的结构基础。使用1-氯-2,4-二硝基苯和还原型谷胱甘肽(GSH)作为Sj26GST底物,显示出EA通过IC 50可抑制Sj26GST活性66%为2.4μM。荧光光谱表明,EA改变了内在色氨酸的环境极性,并且EA降低了Sj26GST与8-苯胺-1-萘磺酸盐(ANS)之间的相互作用(呈剂量依赖性),后者是已知的GST H位点配体。热力学研究表明,Sj26GST和EA之间的相互作用是自发的(ΔG = -29.88±0.07 kJ / mol),是由焓驱动的(ΔH = -9.48±0.42 kJ / mol),且熵变良好(ΔS= 20.40±0.08kJ / mol / K),并且每个Sj26GST二聚体结合了四个EA分子的化学计量。与GSH和EA络合的1.53Å分辨率Sj26GST晶体结构(P 21 21 21空间群)表明,EA主要在二聚体界面结合,并在很大程度上由范德华力和H键稳定。此外,EA结合的H-位点和从ε-NH小于3.5埃附近2 GSH的γ谷氨酰部分的,在每个子单元。











































 京公网安备 11010802027423号
京公网安备 11010802027423号