Biomaterials Advances ( IF 5.5 ) Pub Date : 2020-09-18 , DOI: 10.1016/j.msec.2020.111527 Jianhong Liao , Haisheng Peng , Can Liu , Dan Li , Yihua Yin , Bo Lu , Hua Zheng , Qun Wang
|
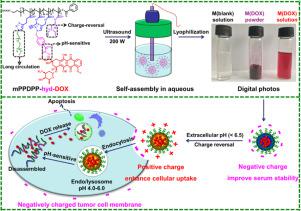
A novel nanodrug delivery system (NDDS) based on block copolymers of Poly(DEA)-block-Poly(PgMA) (PDPP) was developed to enhance in vitro cellular uptake and anticancer efficacy. pH-responsive doxorubicin (DOX) based small molecule prodrug (DOX-hyd-N3) and mPEG-N3 were co-conjugated onto PDPP via copper-catalyzed “Click chemistry” to give a dual pH-responsive polymeric prodrug (mPEG-g-PDPP-g-hyd-DOX), which could be self-assembled into core-shell polymeric micelles (M(DOX)) with particles size of 81 ± 1 nm in aqueous phase. Additionally, the pH-responsive charge-reversal, stability and drug release behaviour at different pHs were then evaluated. Moreover, the surface charge of M(DOX) could quickly convert from negative (- 6.64 ± 3.37 mV) to positive (5.35 ± 1.33 mV) thanks to the protonation of Poly(DEA) moieties as the pH value decreased from 7.4 during blood circulation to 6.5 in extracellular of tumour tissues. Meanwhile, according to the cytotoxicity determined by CCK-8 assay, cellular uptake, flow-cytometric and apoptosis profiles of two human cancer cell lines (HeLa and SW480), we could draw the conclusion that the cellular uptake and anticancer efficacy were significantly enhanced when cells were incubated with micelles at pH 6.5 due to the charge-reversal of micelles from negative to positive. With the protonation of Poly(DEA) moieties in acidic extracellular microenvironment and the pH-responsive DOX release with hydrazone linkage in endo/lysosome pH, this dual pH-responsive-charge-reversal micelle platform might become an encouraging strategy for more effective cancer treatment.
中文翻译:

双重pH响应电荷逆转胶束平台可增强抗癌治疗
开发了一种基于聚(DEA)-嵌段-聚(PgMA)(PDPP)嵌段共聚物的新型纳米药物递送系统(NDDS),以增强体外细胞摄取和抗癌功效。通过铜催化的“点击化学”将基于pH响应阿霉素(DOX)的小分子前药(DOX-hyd-N 3)和mPEG-N 3共缀合到PDPP上,从而制得双重pH响应的聚合物前药(mPEG-克-PDPP-克-hyd-DOX),可以自组装为水相中粒径为81±1 nm的核-壳型聚合物胶束(M(DOX))。另外,然后评估了在不同pH下的pH响应性电荷反转,稳定性和药物释放行为。此外,由于Poly(DEA)部分的质子化,血液循环期间pH值从7.4降低,因此M(DOX)的表面电荷可以迅速从负(-6.64±3.37 mV)转换为正(5.35±1.33 mV)。到肿瘤组织细胞外的6.5。同时,根据CCK-8分析确定的细胞毒性,两种人类癌细胞系(HeLa和SW480)的细胞摄取,流式细胞仪和凋亡图谱,我们可以得出这样的结论:当细胞与胶束在pH 6.5下孵育时,由于胶束的电荷从负向正转换,细胞的吸收和抗癌功效显着增强。随着酸性细胞外微环境中Poly(DEA)部分的质子化以及内endo /溶酶体pH中具有键的pH响应DOX释放,这种双重pH响应电荷逆转胶束平台可能成为鼓励更有效治疗癌症的令人鼓舞的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号