Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2020-09-18 , DOI: 10.1016/j.jwpe.2020.101636 Zhuang Lin , Wenlei Qin , Lei Sun , Xiangjuan Yuan , Dongsheng Xia
|
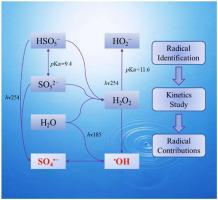
The degradation of Bisphenol A (BPA) in the vacuum ultraviolet/ ultraviolet/ peroxymonosulfate (VUV/UV/PMS) process based on a mini-fluidic VUV/UV photoreaction system has been comprehensively investigated focusing on the degradation performances and kinetics in this study. With 20 min reaction at pH 7.0 in VUV/UV/PMS process, the removal efficiency and the apparent degradation rate constant (kapp) of BPA were > 95.2 % and 0.145 min−1 (R2 = 0.9935). In addition, the impacts of operational parameters and co-existing constituents on BPA degradation are systematically evaluated. Importantly, the effective degradation of BPA (> 96.1 % at least) achieved at the test pH conditions (5–11) after 30 min reaction and achieved the highest BPA removal rate at pH = 11 (0.243 min−1, R2 = 0.9952). The electron paramagnetic resonance and radical scavenger experiments confirmed that both hydroxyl radical ( OH) and sulfate radical (SO4
OH) and sulfate radical (SO4 −) are the predominant reactive radicals in VUV/UV/PMS process. The second-order reaction rate constants of BPA with
−) are the predominant reactive radicals in VUV/UV/PMS process. The second-order reaction rate constants of BPA with  OH and SO4
OH and SO4 − under different pH conditions were determined to be 0.74–1.86 × 1010 M−1 s−1 and 2.92–5.01 × 109 M−1 s−1 via the competition kinetics. Meanwhile, the relative contribution rate of
− under different pH conditions were determined to be 0.74–1.86 × 1010 M−1 s−1 and 2.92–5.01 × 109 M−1 s−1 via the competition kinetics. Meanwhile, the relative contribution rate of  OH decreased from 97.2 %–33.1 % with the increasement of pH from 5.0 to 11.0, while the relative contribution rate of SO4
OH decreased from 97.2 %–33.1 % with the increasement of pH from 5.0 to 11.0, while the relative contribution rate of SO4 − rose from 1.7 % to 48.9 %. The present study demonstrated that the VUV/UV/PMS process, as a new advanced oxidation process, has potential applications for micropollutants removal in water.
− rose from 1.7 % to 48.9 %. The present study demonstrated that the VUV/UV/PMS process, as a new advanced oxidation process, has potential applications for micropollutants removal in water.
中文翻译:

VUV / UV /过氧一硫酸盐体系中硫酸根和羟基自由基诱导双酚A降解的动力学和机理
在本研究中,着重研究了双酚A(BPA)在基于微流体VUV / UV光反应体系的真空紫外/紫外/过氧单硫酸盐(VUV / UV / PMS)过程中的降解。在VUV / UV / PMS过程中,在pH 7.0下反应20分钟,BPA的去除效率和表观降解速率常数(k app)分别> 95.2%和0.145 min -1(R 2= 0.9935)。此外,系统地评估了操作参数和共存成分对双酚A降解的影响。重要的是,反应30分钟后,在测试pH条件(5-11)下BPA的有效降解(至少> 96.1%)达到了,并且在pH = 11时BPA去除率最高(0.243 min -1,R 2 = 0.9952) )。电子顺磁共振和自由基清除剂的实验证实,这两个羟基自由基( OH)和硫酸根(SO 4 - )是在VUV / UV / PMS过程的主要反应性自由基。与BPA的二阶反应速率常数OH和SO 4 - ,测定不同pH条件下为0.74-1.86×10
OH)和硫酸根(SO 4 - )是在VUV / UV / PMS过程的主要反应性自由基。与BPA的二阶反应速率常数OH和SO 4 - ,测定不同pH条件下为0.74-1.86×10

 10 M -1 s -1和2.92–5.01×10 9 M -1 s -1通过竞争动力学。同时,的相对贡献率
10 M -1 s -1和2.92–5.01×10 9 M -1 s -1通过竞争动力学。同时,的相对贡献率 OH从97.2%随着pH的increasement从5.0至11.0下降-33.1%,而SO的相对贡献率4 -玫瑰从1.7%至48.9%。本研究表明,VUV / UV / PMS工艺作为一种新的高级氧化工艺,在去除水中的微污染物方面具有潜在的应用前景。
OH从97.2%随着pH的increasement从5.0至11.0下降-33.1%,而SO的相对贡献率4 -玫瑰从1.7%至48.9%。本研究表明,VUV / UV / PMS工艺作为一种新的高级氧化工艺,在去除水中的微污染物方面具有潜在的应用前景。









































 京公网安备 11010802027423号
京公网安备 11010802027423号