Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.molliq.2020.114359 Qi Xiao , Jiandan Liang , Huajian Luo , Haimei Li , Jing Yang , Shan Huang
|
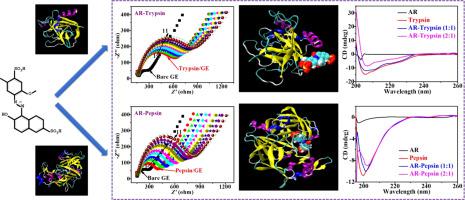
Herein, binding interactions of food colourant allura red (AR) with trypsin and pepsin were comparably investigated for deep revelations of conformational structures and activities of proteinases affected by food colourant. Various results indicated that one AR bound with one proteinase to form novel ground state complex under the binding forces of van der Waal interactions and hydrogen bonds. Intrinsic fluorescence of proteinases was quenched by AR via static fluorescence quenching mode. Conformational structures of proteinases were all changed obviously after their binding interactions with AR, resulting in their structure transformation to the β-sheet structure. AR bound with the allosteric site of proteinases to inhibit their activities via non-competitive manner. Finally, AR protected human serum albumin from the digestion of proteinases efficiently. These results revealed the exact binding mechanisms of food colourant AR with proteinases, which illuminated the possible biological risk of food colourant AR on human beings.
中文翻译:

食品色素变色红对胰蛋白酶和胃蛋白酶构象结构和活性的影响
在本文中,比较研究了食用色素变色红(AR)与胰蛋白酶和胃蛋白酶的结合相互作用,以揭示受食用色素影响的构象结构和蛋白酶活性的深入揭示。各种结果表明,一种AR与一种蛋白酶结合,在范德华相互作用和氢键的结合力作用下形成新的基态复合物。通过静态荧光淬灭模式通过AR淬灭蛋白酶的内在荧光。蛋白酶与AR的结合相互作用后,其构象结构均发生了明显变化,导致其结构转化为β页结构。AR与蛋白酶的变构位点结合,通过非竞争性方式抑制其活性。最后,AR有效地保护了人血清白蛋白免于蛋白酶的消化。这些结果揭示了食用色素AR与蛋白酶的确切结合机制,这阐明了食用色素AR对人类的潜在生物学风险。









































 京公网安备 11010802027423号
京公网安备 11010802027423号