Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.molliq.2020.114357 Reza Tayebee , Atefeh H. Nasr
|
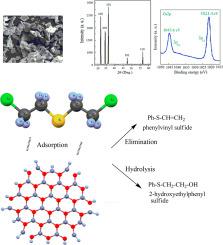
Sulfur mustard is a strong vesicant, leading to severe skin damages as well as eye blistering and may lead to death if inhaled. Unfortunately, there are no effective treatments for mustard-induced injury. Hence, there is considerable attention in the development of materials that can absorb or detoxify mustard gas. In the first part of this report, detoxification of sulfur mustard is performed on the synthetic ZnO nanosheets. The intended ZnO nanostructure is prepared via a simple and efficient method and characterized by means of FT-IR, XRD, FESEM, and XPS to confirm formation of the desired nanosheets. Then, detoxification and degradation of sulfur mustard is carried out on the prepared ZnO nanostructures at room temperature and a good rate for sulfur mustard detoxification was attained at the initial stages of the reaction (1−12 h) with a rate constant of 0.081 h−1 and a half-life of 8.4 h. At the second part of the present study, the density functional calculations including B3LYP and wB97XD are used to monitor the adsorption of this toxic material on the surface of various ZnO nanoclusters (ZnONCs). The values of adsorption energy of mustard gas are determined in the range of about −16 to −54 kcal/mol with enthalpies in the range of about −15 to-51 kcal/mol and the Gibbs free energies in the range of −3 to −28 kcal/mol at 298 K based on B3LYP level of theory. The geometry parameters are calculated for all geometries by consideration of the charge analysis and frontier molecular orbitals. Findings revealed that these systems are useful chemical sensors for sulfur mustard. Moreover, it is found that the CCL-ZnONS can effectively interact with this material and modifies the sensitivity of zinc oxide towards this agent.
中文翻译:

研究芥菜化学战在ZnO纳米结构上的吸附和解毒
芥末硫磺是强力的起泡剂,会导致严重的皮肤损伤以及眼疱,如果吸入会导致死亡。不幸的是,目前还没有有效的方法治疗芥子气引起的损伤。因此,在可吸收或解毒芥子气的材料的开发中引起了极大的关注。在本报告的第一部分中,对合成的ZnO纳米片进行了硫芥菜的解毒。所需的ZnO纳米结构是通过简单有效的方法制备的,并通过FT-IR,XRD,FESEM和XPS进行表征,以确认所需纳米片的形成。然后,-1半衰期为8.4小时。在本研究的第二部分,使用密度函数计算(包括B3LYP和wB97XD)来监视这种有毒物质在各种ZnO纳米团簇(ZnONCs)表面上的吸附。芥子气的吸附能值在约-16至-54 kcal / mol的范围内确定,焓在约-15至-51 kcal / mol的范围内,吉布斯自由能在-3至根据B3LYP理论水平,在298 K时为−28 kcal / mol。通过考虑电荷分析和前沿分子轨道来计算所有几何形状的几何参数。研究结果表明,这些系统对于硫芥末是有用的化学传感器。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号