European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.ejmech.2020.112848 Junsong Guo , Bingyi Chen , Ying Yu , Bao Cheng , Yingchen Ju , Jieyu Tang , Zhengjun Cai , Qiong Gu , Jun Xu , Huihao Zhou
|
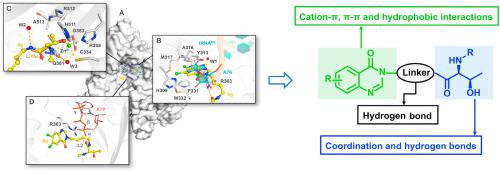
Aminoacyl-tRNA synthetases (aaRSs) are an attractive class of antibacterial drug targets due to their essential roles in protein translation. While most traditional aaRS inhibitors target the binding pockets of substrate amino acids and/or ATP, we recently developed a class of novel tRNA-amino acid dual-site inhibitors including inhibitor 3 ((2S,3R)-2-amino-N-((E)-4-(6,7-dichloro-4-oxoquinazolin-3(4H)-yl)but-2-en-1-yl)-3-hydroxybutanamide) against threonyl-tRNA synthetase (ThrRS). Here, the binding modes and structure-activity relationships (SARs) of these inhibitors were analyzed by the crystal structures of Salmonella enterica ThrRS (SeThrRS) in complex with three of them. Based on the cocrystal structures, twelve quinazolinone-threonine hybrids were designed and synthesized, and their affinities, enzymatic inhibitory activities, and cellular potencies were evaluated. The best derivative 8g achieved a Kd value of 0.40 μM, an IC50 value of 0.50 μM against SeThrRS and MIC values of 16∼32 μg/mL against the tested bacterial strains. The cocrystal structure of the SeThrRS-8g complex revealed that 8g induced a bended conformation for Met332 by forming hydrophobic interactions, which better mimicked the binding of tRNAThr to ThrRS. Moreover, the inhibitory potency of 8g was less impaired than a reported ATP competitive inhibitor at high concentrations of ATP, supporting our hypothesis that tRNA site inhibitors are likely superior to ATP site inhibitors in vivo, where ATP typically reaches millimolar concentrations.
中文翻译:

一类喹唑啉酮-苏氨酸杂化物作为抗菌ThrRS抑制剂的结构导向优化及机理研究
氨酰基-tRNA合成酶(aaRSs)由于其在蛋白质翻译中的重要作用而成为一类有吸引力的抗菌药物靶标。虽然大多数传统的aaRS抑制剂都靶向底物氨基酸和/或ATP的结合口袋,但我们最近开发了一类新型tRNA-氨基酸双位抑制剂,包括抑制剂3((2 S,3 R)-2-amino- N -((E)-4-(6,7-dichloro-4-oxoquinazolin-3(4 H)-yl)but-2-en-1-yl)-3-hydroxybutanamide)反对苏氨酸tRNA合成酶(ThrRS) 。在此,通过肠沙门氏菌ThrRS(Se)的晶体结构分析了这些抑制剂的结合模式和构效关系(SARs)。ThrRS)与其中三个复杂。基于共晶体结构,设计并合成了十二种喹唑啉酮-苏氨酸杂化物,并评估了它们的亲和力,酶抑制活性和细胞效能。最好的衍生物8g的K d值为0.40μM,对Se ThrRS的IC 50值为0.50μM,对测试的细菌菌株的MIC值为16〜32μg/ mL。Se ThrRS- 8g复合物的共晶体结构显示8g通过形成疏水相互作用诱导Met332的弯曲构象,从而更好地模拟了tRNA Thr与ThrRS的结合。而且,其的抑制力与8g相比,在高浓度的ATP竞争性抑制剂作用下,其8g的受损程度要小一些,这支持了我们的假设,即体内tRNA位置抑制剂可能优于ATP位置抑制剂,而ATP通常在体内达到毫摩尔浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号