当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the functionality of the N-terminal domain in xylanase 10A from Ruminococcus albus 8
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.enzmictec.2020.109673 Alem Storani 1 , Sergio A Guerrero 1 , Alberto A Iglesias 1
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.enzmictec.2020.109673 Alem Storani 1 , Sergio A Guerrero 1 , Alberto A Iglesias 1
Affiliation
|
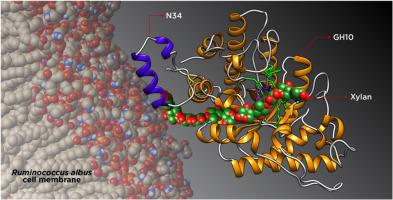
We analyzed the structure to function relationships in Ruminococcus albus 8 xylanase 10A (RalXyn10A) finding that the N-terminus 34-amino acids sequence (N34) in the protein is particularly functional. We performed the recombinant wild type enzyme's characterization and that of the truncated mutant lacking the N34 extreme (RalΔN34Xyn10A). The truncated enzyme exhibited about half of the activity and reduced affinity for binding to insoluble saccharides. These suggest a (CBM)-like function for the N34 motif. Besides, RalXyn10A activity was diminished by redox agent dithiothreitol, a characteristic absent in RalΔN34Xyn10A. The N34 sequence exhibited a significant similarity with protein components of the ABC transporter of the bacterial membrane, and this motif is present in other proteins of R. albus 8. Data suggest that N34 would confer RalXyn10A the capacity to interact with polysaccharides and components of the cell membrane, enhancing the degradation of the substrate and uptake of the products by the bacterium.
中文翻译:

关于白色瘤胃球菌 8 木聚糖酶 10A 中 N 端结构域的功能
我们分析了 Ruminococcus albus 8 木聚糖酶 10A (RalXyn10A) 中的结构与功能关系,发现该蛋白质中的 N 末端 34 个氨基酸序列 (N34) 特别具有功能。我们进行了重组野生型酶的表征和缺乏 N34 极端 (RalΔN34Xyn10A) 的截短突变体的表征。截短的酶表现出大约一半的活性并且降低了与不溶性糖类结合的亲和力。这些表明 N34 基序具有类似 (CBM) 的功能。此外,氧化还原剂二硫苏糖醇降低了 RalXyn10A 的活性,这是 RalΔN34Xyn10A 中不存在的特征。N34 序列与细菌膜的 ABC 转运蛋白的蛋白质组分表现出显着的相似性,并且该基序存在于 R. albus 8 的其他蛋白质中。
更新日期:2020-12-01
中文翻译:

关于白色瘤胃球菌 8 木聚糖酶 10A 中 N 端结构域的功能
我们分析了 Ruminococcus albus 8 木聚糖酶 10A (RalXyn10A) 中的结构与功能关系,发现该蛋白质中的 N 末端 34 个氨基酸序列 (N34) 特别具有功能。我们进行了重组野生型酶的表征和缺乏 N34 极端 (RalΔN34Xyn10A) 的截短突变体的表征。截短的酶表现出大约一半的活性并且降低了与不溶性糖类结合的亲和力。这些表明 N34 基序具有类似 (CBM) 的功能。此外,氧化还原剂二硫苏糖醇降低了 RalXyn10A 的活性,这是 RalΔN34Xyn10A 中不存在的特征。N34 序列与细菌膜的 ABC 转运蛋白的蛋白质组分表现出显着的相似性,并且该基序存在于 R. albus 8 的其他蛋白质中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号