Energy Storage Materials ( IF 18.9 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.ensm.2020.09.006 Chuanliang Wei , Liwen Tan , Yuan Tao , Yongling An , Yuan Tian , Huiyu Jiang , Jinkui Feng , Yitai Qian
|
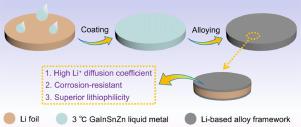
Lithium (Li) metal is a promising anode for next-generation high-energy-density lithium-ion batteries (LIBs). Nevertheless, the stability of Li-metal anode is poor due to the severe corrosion by liquid electrolyte, uncontrollable growth of Li dendrites, huge volume expansion, and unstable solid electrolyte interphase (SEI). The high chemical reactivity of Li metal is the main inducement for the unstability of Li-metal anode. Herein, the stability of Li-metal anode is improved by passivating its surface with 3°C GaInSnZn liquid metal (LM). A Li-based alloy framework with submicron-scale grains is formed on the surface of Li metal through the spontaneous reaction between metallic Li and LM at room temperature. The Li-based alloy framework is tightly attached on Li metal without exfoliation and mechanical rupture even under bending and folding. The framework has higher Li+ diffusion coefficient, lower chemical reactivity, and better lithiophilicity than pure Li. Under the regulation of the multifunctional framework, the corrosion, uneven Li deposition, and unstable interface are effectively relieved even in a more corrosive carbonate-based electrolyte. When paired with 5 V-class cathodes, the full cells with passivated Li-metal anodes exhibit superior electrochemical performances. This passivation strategy also shows great potentials for high-reactive Na-metal and K-metal anodes.
中文翻译:

室温液态金属的界面钝化可实现稳定的5 V级锂金属电池在商业碳酸盐基电解液中的使用
锂(Li)金属是下一代高能量密度锂离子电池(LIB)的有希望的阳极。然而,由于液体电解质的严重腐蚀,锂树枝状晶体的不可控制的生长,巨大的体积膨胀以及不稳定的固体电解质中间相(SEI),锂金属阳极的稳定性很差。锂金属的高化学反应性是导致锂金属阳极不稳定的主要原因。在此,通过用3℃的GaInSnZn液态金属(LM)钝化其表面来提高锂金属阳极的稳定性。通过在室温下金属Li和LM之间的自发反应,在Li金属表面形成具有亚微米级晶粒的Li基合金骨架。锂基合金框架牢固地附着在锂金属上,即使在弯曲和折叠下也不会脱落和机械断裂。+扩散系数,较低的化学反应性和比纯Li更好的亲硫性。在多功能框架的调节下,即使在腐蚀性更强的碳酸盐基电解质中,也可有效缓解腐蚀,不均匀的锂沉积和不稳定的界面。与5个V级阴极配对时,带有钝化锂金属阳极的全电池表现出优异的电化学性能。这种钝化策略还显示出高反应性的Na-金属和K-金属阳极的巨大潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号