当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the flocculation mechanism of quartz and kaolinite with polyacrylamide in seawater: A molecular dynamics approach
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125576 Gonzalo R. Quezada , Matías Jeldres , Norman Toro , Pedro Robles , Pedro G. Toledo , Ricardo I. Jeldres
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125576 Gonzalo R. Quezada , Matías Jeldres , Norman Toro , Pedro Robles , Pedro G. Toledo , Ricardo I. Jeldres
|
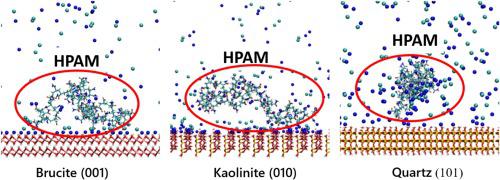
Abstract The scarcity of water resources for mining activities drives the search for new low-quality water sources such as well-water and seawater. Seawater was found to be a promising alternative, but it may pose significant operational challenges, for example, when it needs to be recovered from the tailings in thickening stages for subsequent recycling. This is mainly explained by the high saline environment and colloidal magnesium precipitates that are generated at highly alkaline conditions. In this work, we use molecular dynamics (MD) simulations to understand the affinity of the flocculant with colloidal magnesium precipitates and the main minerals that make up a mining tailing such as quartz and kaolinite. The results are contrasted with the in-situ characterization of aggregates through the Focused Beam Reflectance Measurement (FBRM). Through X-ray diffraction, it was found that the magnesium precipitates are mainly composed of brucite crystals. The MD results allowed to explain the experimental results, mainly when solid magnesium precipitates appear at high pH, where the flocculant loses its effectiveness sharply. This is related to the undesired association among the flocculant with brucite. The adsorption is mainly carried out by the interaction between the deprotonated oxygen from the acrylic group of the polymer and the oxygen from the hydroxide of the brucite surface. There is also a significant contribution of hydrogen bonding between nitrogen from the acrylamide group and oxygen from the hydroxide.
中文翻译:

了解石英和高岭石与聚丙烯酰胺在海水中的絮凝机制:分子动力学方法
摘要 采矿活动水资源的稀缺促使人们寻找新的低质量水源,如井水和海水。海水被发现是一种很有前途的替代品,但它可能会带来重大的运营挑战,例如,当需要在浓缩阶段从尾矿中回收海水以进行后续回收时。这主要是由高盐环境和在高碱性条件下产生的胶态镁沉淀物来解释的。在这项工作中,我们使用分子动力学 (MD) 模拟来了解絮凝剂与胶体镁沉淀物和构成采矿尾矿的主要矿物(如石英和高岭石)的亲和力。结果与通过聚焦光束反射测量 (FBRM) 对聚集体的原位表征进行对比。通过X射线衍射发现,镁沉淀物主要由水镁石晶体组成。MD 结果可以解释实验结果,主要是当固体镁沉淀出现在高 pH 值时,絮凝剂会急剧失去其效力。这与絮凝剂与水镁石之间的不良关联有关。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。主要是当固体镁沉淀出现在高 pH 值时,絮凝剂会急剧失去其效力。这与絮凝剂与水镁石之间的不良关联有关。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。主要是当固体镁沉淀出现在高 pH 值时,絮凝剂会急剧失去其效力。这与絮凝剂与水镁石之间的不良关联有关。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。
更新日期:2021-01-01
中文翻译:

了解石英和高岭石与聚丙烯酰胺在海水中的絮凝机制:分子动力学方法
摘要 采矿活动水资源的稀缺促使人们寻找新的低质量水源,如井水和海水。海水被发现是一种很有前途的替代品,但它可能会带来重大的运营挑战,例如,当需要在浓缩阶段从尾矿中回收海水以进行后续回收时。这主要是由高盐环境和在高碱性条件下产生的胶态镁沉淀物来解释的。在这项工作中,我们使用分子动力学 (MD) 模拟来了解絮凝剂与胶体镁沉淀物和构成采矿尾矿的主要矿物(如石英和高岭石)的亲和力。结果与通过聚焦光束反射测量 (FBRM) 对聚集体的原位表征进行对比。通过X射线衍射发现,镁沉淀物主要由水镁石晶体组成。MD 结果可以解释实验结果,主要是当固体镁沉淀出现在高 pH 值时,絮凝剂会急剧失去其效力。这与絮凝剂与水镁石之间的不良关联有关。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。主要是当固体镁沉淀出现在高 pH 值时,絮凝剂会急剧失去其效力。这与絮凝剂与水镁石之间的不良关联有关。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。主要是当固体镁沉淀出现在高 pH 值时,絮凝剂会急剧失去其效力。这与絮凝剂与水镁石之间的不良关联有关。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。吸附主要通过来自聚合物丙烯酸基团的去质子化氧与来自水镁石表面氢氧化物的氧之间的相互作用进行。来自丙烯酰胺基团的氮和来自氢氧化物的氧之间的氢键也有重要贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号