当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancement of Cu(II) removal by carbon disulfide modified black wattle tannin gel
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125594 Xubing Sun , Jiayong Zhang , Yaohui You
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.colsurfa.2020.125594 Xubing Sun , Jiayong Zhang , Yaohui You
|
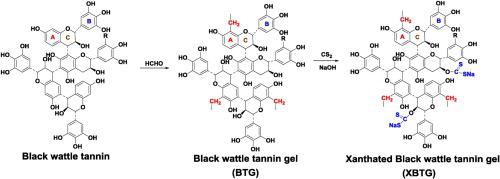
Abstract Xanthated black wattle tannin gel (XBTG) was prepared by modifying black wattle tannin gel (BTG) with carbon disulfide to improve the performance of Cu(II) removal. Structure characterization demonstrated that xanthate groups were successfully introduced onto BTG and surface morphology of BTG was maintained after CS2-modification. Zeta potential measurement showed that XBTG was negative charged at a pH range from 1 to 5. Compared to the BTG, the thermal stability and the surface area of XBTG decreased, but the Cu(II) adsorption rate and adsorption capacity increased. The adsorption kinetics of BTG and XBTG followed pseudo-second-order model. The adsorption isotherms fitted well with Langmuir model, and the calculated maximum adsorption capacity for the XBTG increased to 149.25 mg/g at 298 K, which was 228.95 % higher than BTG. Moreover, the Cu(II) loaded XBTG could be regenerated using 0.1 M ethylenediaminetetraacetic acid disodium salt solution. The adsorption capacity of the regenerated XBTG after five cycles decreased to 58 % compared with the fresh adsorbent, which was equivalent to that of BTG. The adsorption mechanism was attributed to the surface complexation of Cu(II) by adjacent phenolic hydroxyl groups and xanthate groups or to the ion exchange between Na(I) and Cu(II).
中文翻译:

二硫化碳改性黑荆树单宁凝胶对Cu(II)去除的增强作用
摘要 用二硫化碳对黑荆树单宁凝胶(BTG)进行改性,以提高对Cu(II)的去除性能,制备黄原黑荆树单宁凝胶(XBTG)。结构表征表明黄原酸基团被成功地引入到 BTG 上,并且在 CS2 改性后保持了 BTG 的表面形态。Zeta 电位测量表明,XBTG 在 1 到 5 的 pH 范围内带负电荷。与 BTG 相比,XBTG 的热稳定性和表面积降低,但 Cu(II) 吸附率和吸附容量增加。BTG和XBTG的吸附动力学遵循拟二级模型。吸附等温线与朗缪尔模型拟合良好,计算得到的 XBTG 在 298 K 时的最大吸附容量增加到 149.25 mg/g,比 BTG 高 228.95%。而且,使用 0.1 M 乙二胺四乙酸二钠盐溶液可以再生负载 Cu(II) 的 XBTG。与新鲜吸附剂相比,5 个循环后再生的 XBTG 的吸附容量下降到 58%,与 BTG 的吸附容量相当。吸附机制归因于相邻酚羟基和黄原酸酯基团对 Cu(II) 的表面络合或 Na(I) 和 Cu(II) 之间的离子交换。
更新日期:2021-01-01
中文翻译:

二硫化碳改性黑荆树单宁凝胶对Cu(II)去除的增强作用
摘要 用二硫化碳对黑荆树单宁凝胶(BTG)进行改性,以提高对Cu(II)的去除性能,制备黄原黑荆树单宁凝胶(XBTG)。结构表征表明黄原酸基团被成功地引入到 BTG 上,并且在 CS2 改性后保持了 BTG 的表面形态。Zeta 电位测量表明,XBTG 在 1 到 5 的 pH 范围内带负电荷。与 BTG 相比,XBTG 的热稳定性和表面积降低,但 Cu(II) 吸附率和吸附容量增加。BTG和XBTG的吸附动力学遵循拟二级模型。吸附等温线与朗缪尔模型拟合良好,计算得到的 XBTG 在 298 K 时的最大吸附容量增加到 149.25 mg/g,比 BTG 高 228.95%。而且,使用 0.1 M 乙二胺四乙酸二钠盐溶液可以再生负载 Cu(II) 的 XBTG。与新鲜吸附剂相比,5 个循环后再生的 XBTG 的吸附容量下降到 58%,与 BTG 的吸附容量相当。吸附机制归因于相邻酚羟基和黄原酸酯基团对 Cu(II) 的表面络合或 Na(I) 和 Cu(II) 之间的离子交换。











































 京公网安备 11010802027423号
京公网安备 11010802027423号