Chemosphere ( IF 8.1 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.chemosphere.2020.128386 Yinian Zhu , Wanying Wei , Shen Tang , Zongqiang Zhu , Qiming Yan , Lihao Zhang , Huan Deng
|
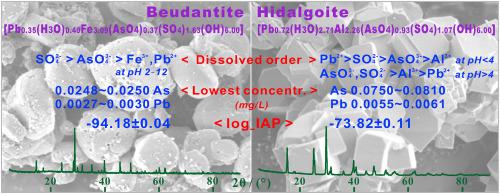
Beudantite and hidalgoite were synthesized and characterized to investigate their possible immobilization for arsenic and lead in acidic and oxidizing environments by a long-term dissolution. The synthetic beudantite [Pb0.35(H3O)0.40Fe3.09(AsO4)0.37(SO4)1.63(OH)6.00] was spherulitic pseudo-cubic crystals with nearly smooth surface. The synthetic hidalgoite [Pb0.72(H3O)2.71Al2.26(AsO4)0.93(SO4)1.07(OH)6.00] was well-formed pseudo-cubic, pseudo-cuboctahedral or pseudo-octahedral crystals. During the beudantite dissolution, the constituents were dissolved preferentially in the order of SO42- > AsO43- > Pb2+ > Fe3+ in the early 24 h and SO42- > AsO43- > Fe3+ > Pb2+ after 24 h; the dissolved concentrations exhibited a minimum of 0.0027∼0.0030 mg/L Pb and 0.0248∼0.0250 mg/L As. During the hidalgoite dissolution, the constituents were dissolved preferentially in the order of Pb2+ > SO42- > AsO43- > Al3+ at initial pH<4 or AsO43-,SO42- > Al3+ > Pb2+ at initial pH>4; the dissolved concentrations showed a minimum of 0.0055∼0.0061 mg/L Pb and 0.0750∼0.0810 mg/L As. From the data of the dissolution at initial pH 2 and 25 °C for 270∼330 d, the ion-activity products [logˍIAP] were estimated to be -94.18±0.04 for the beudantite and -73.82±0.11 for the hidalgoite, respectively. The concentrations of Pb and As released in the beudantite dissolution were always lower than in the hidalgoite dissolution and arsenate appeared to be much more soluble than Pb. Beudantite was more effective for the immobilization of As and Pb than hidalgoite.
中文翻译:

pH 2-12和25-45°C下可能长期同时固定砷和铅时,膨润土和锂辉石的溶解和稳定性的比较研究
合成了膨润土和水辉石,并对其进行了表征,以研究其在长期溶解下在酸性和氧化性环境中对砷和铅的固定作用。合成的膨润土[Pb 0.35(H 3 O)0.40 Fe 3.09(AsO 4)0.37(SO 4)1.63(OH)6.00 ]是球状伪立方晶体,表面几乎光滑。合成方铁矿[Pb 0.72(H 3 O)2.71 Al 2.26(AsO 4)0.93(SO 4)1.07(OH)6.00 ]为格式良好的伪立方,伪立方八面体或伪八面体晶体。在膨润土溶解过程中,成分在24小时内优先以SO 4 2- > AsO 4 3- > Pb 2+ > Fe 3+和SO 4 2- > AsO 4 3- > Fe 3+的顺序优先溶解。24小时后> Pb 2+;溶解浓度最低为0.0027〜0.0030 mg / L Pb,最小为0.0248〜0.0250 mg / L As。在菱铁矿溶解过程中,各组分按照Pb 2+ > SO 4 2- > AsO 4的顺序优先溶解3- >初始pH <4时为Al 3+或AsO 4 3-,初始pH> 4时为SO 4 2- > Al 3+ > Pb 2+;溶解浓度最低为0.0055〜0.0061 mg / L Pb,最小为0.0750〜0.0810 mg / L As。根据初始pH 2和25°C下270至330 d时的溶出度数据,据估计,对于膨润土,离子活性产物[logˍIAP]为-94.18±0.04,对于锂蒙脱石,其离子活性产物[logˍIAP]分别为-73.82±0.11。球铁矿溶解中释放的Pb和As的浓度总是低于菱铁矿溶解中的浓度,并且砷酸盐似乎比Pb溶解性高得多。膨润土对固定化As和Pb的作用比菱铁矿更有效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号