Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.cplett.2020.138016 Yu Zhang , Jie Li , Yang Gong , Hui Guo , Hongliang Zhang , Ke Du , Jingkun Wang
|
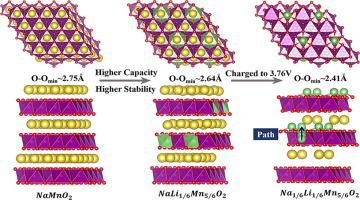
Through the calculation of P3-type Na2/3+xLi1/6Mn5/6O2 based on density function theory (DFT), the arrangement of Li/Mn is reasonably determined. In addition, Li-ion is observed to migrate from the TM(transition metal) layer to the AM(alkali metal) layer during the discharge process, and the O-O bond length becomes significantly shorter (~2.41 Å) which suggests peroxo-like O-O dimers are formed. After the calculation of the O2 evolution potential (>2.11 eV), it is found that O2– would not become O2 to escape after the transformation from O2– to peroxide pair, which suggests that the redox of O is reversible.
中文翻译:

用密度泛函理论探索P3型Na 2/3 + x Li 1/6 Mn 5/6 O 2正极材料中氧阴离子电荷补偿机理
通过基于密度泛函理论(DFT)的P3型Na 2/3 + x Li 1/6 Mn 5/6 O 2的计算,合理确定了Li / Mn的排列。此外,在放电过程中观察到锂离子从TM(过渡金属)层迁移到AM(碱金属)层,OO键长度显着缩短(〜2.41Å),这表明类似过氧的OO形成二聚体。在计算出O 2的析出电位(> 2.11 eV)后,发现O 2–在从O 2–转变为过氧化物对后不会变成O 2逃逸,这表明O的氧化还原是可逆的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号