Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.cej.2020.127052 Xinxin Long , Shengjiong Yang , Xiaojie Qiu , Dahu Ding , Chuanping Feng , Rongzhi Chen , JihuaTan , Xinming Wang , Nan Chen , Qin Lei
|
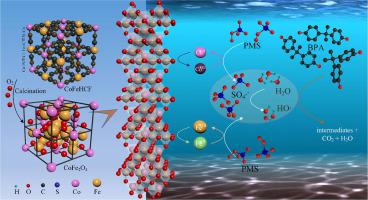
A facile coprecipitation-thermal method is reported to prepare spinel cobalt ferrites CoFe2O4 nanoparticles (NPs) with a well-defined mesoporous dominated structure using hybrid cobalt-ion hexacyanoferrate (CoFeHCF) NPs as a template. The properties of the synthesized catalysts are evidenced by various characterization methods. And the effects of time, pH, catalyst/oxidant/BPA dosage, coexisting ions and natural organic matter (NOM) on catalytic degradation of bisphenol A (BPA) are investigated. The as-prepared CoFe2O4 shows a high catalytic performance of 97% elimination for 45 μM BPA in 40 min. The excellent catalytic performance could be attributed to the large specific surface area (66.18 m2/g) and high content of cobalt (the atomic ratio of Fe/Co is 1.86) of CoFe2O4 NPs. Slightly alkaline pH, higher catalyst dosage and anions including Cl- and CO32-/HCO3- are favorable for BPA degradation. Especially, 81.57% mineralization of BPA could be achieved in 60 min under the optimization system. The catalytic capacity of CoFe2O4 could be regenerated by simple washing and drying at 200℃, which is much lower than the crystal temperature. SO4∙- and HO∙ are the primary reactive species responsible for BPA oxidation while SO4∙- might play a dominant role. This work provides a novel method to produce CoFe2O4 nanocatalysts for contaminant degradation and encourage the extended application of CoFeHCF in the environment.
中文翻译:

钴离子六氰合铁酸杂化纳米粒子衍生的CoFe 2 O 4异构化过氧单硫酸盐对双酚A的降解
报道了一种简便的共沉淀-热法,以混合钴离子六氰铁酸盐(CoFeHCF)NPs为模板,制备了具有定义明确的中孔主导结构的尖晶石钴铁氧体CoFe 2 O 4纳米颗粒(NPs)。合成的催化剂的性质通过各种表征方法证明。考察了时间,pH,催化剂/氧化剂/ BPA用量,共存离子和天然有机物(NOM)对双酚A(BPA)催化降解的影响。所制备的CoFe 2 O 4在40分钟内显示出对45μMBPA的97%消除的高催化性能。优异的催化性能可归因于较大的比表面积(66.18 m 2/ g)和高含量的CoFe 2 O 4 NPs (Fe / Co原子比为1.86)。微碱性pH值,较高的催化剂用量和阴离子含Cl -和CO 3 2- / HCO 3 -是BPA降解有利。特别是,在优化系统下,在60分钟内可以达到81.57%的BPA矿化。CoFe 2 O 4的催化能力可以通过简单的洗涤和在200℃下干燥来再生,这远低于晶体温度。SO 4 ∙ -和HO∙是负责BPA氧化的主要反应性物质,而SO 4 ∙ -可能起主导作用。这项工作提供了一种生产用于污染物降解的CoFe 2 O 4纳米催化剂的新方法,并鼓励了CoFeHCF在环境中的广泛应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号