Cell Stem Cell ( IF 19.8 ) Pub Date : 2020-09-18 , DOI: 10.1016/j.stem.2020.09.013 Jessie Huang 1 , Adam J Hume 2 , Kristine M Abo 1 , Rhiannon B Werder 3 , Carlos Villacorta-Martin 4 , Konstantinos-Dionysios Alysandratos 1 , Mary Lou Beermann 1 , Chantelle Simone-Roach 5 , Jonathan Lindstrom-Vautrin 4 , Judith Olejnik 2 , Ellen L Suder 2 , Esther Bullitt 6 , Anne Hinds 7 , Arjun Sharma 8 , Markus Bosmann 9 , Ruobing Wang 10 , Finn Hawkins 1 , Eric J Burks 11 , Mohsan Saeed 12 , Andrew A Wilson 1 , Elke Mühlberger 2 , Darrell N Kotton 13
|
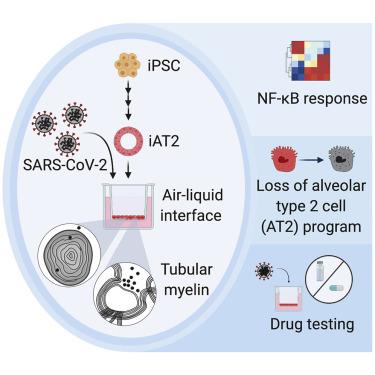
A hallmark of severe COVID-19 pneumonia is SARS-CoV-2 infection of the facultative progenitors of lung alveoli, the alveolar epithelial type 2 cells (AT2s). However, inability to access these cells from patients, particularly at early stages of disease, limits an understanding of disease inception. Here, we present an in vitro human model that simulates the initial apical infection of alveolar epithelium with SARS-CoV-2 by using induced pluripotent stem cell-derived AT2s that have been adapted to air-liquid interface culture. We find a rapid transcriptomic change in infected cells, characterized by a shift to an inflammatory phenotype with upregulation of NF-κB signaling and loss of the mature alveolar program. Drug testing confirms the efficacy of remdesivir as well as TMPRSS2 protease inhibition, validating a putative mechanism used for viral entry in alveolar cells. Our model system reveals cell-intrinsic responses of a key lung target cell to SARS-CoV-2 infection and should facilitate drug development.
中文翻译:

SARS-CoV-2 感染多能干细胞衍生的人肺泡 2 型细胞引发快速上皮内在炎症反应
重症 COVID-19 肺炎的一个标志是肺泡兼性祖细胞(肺泡上皮 2 型细胞 (AT2s))感染 SARS-CoV-2。然而,无法从患者身上获取这些细胞,特别是在疾病的早期阶段,限制了对疾病起始的理解。在这里,我们提出了一种体外人体模型,通过使用已适应气液界面培养的诱导多能干细胞衍生的 AT2 来模拟 SARS-CoV-2 肺泡上皮的初始根尖感染。我们发现受感染细胞的转录组发生快速变化,其特征是向炎症表型转变,并伴有 NF-κB 信号传导上调和成熟肺泡程序的丧失。药物测试证实了瑞德西韦以及 TMPRSS2 蛋白酶抑制的功效,验证了病毒进入肺泡细胞的假定机制。我们的模型系统揭示了关键肺部靶细胞对 SARS-CoV-2 感染的细胞内在反应,应该有助于药物开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号