Catalysis Communications ( IF 3.4 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.catcom.2020.106165 Lei Zheng , Ye Wang , Xianggao Meng , Yunfeng Chen
|
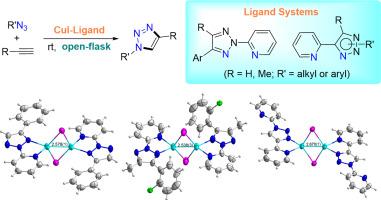
Pyridinyl-triazole ligand systems (including N2-2-pyridinyl 1,2,3-triazoles and N1/N2-substituted 2-(NH-1,2,3-triazol-4-yl)pyridines) were found to be superior ligands for CuI-catalyzed azide-alkyne cycloaddition (CuAAC) reactions. Low catalyst loadings, short reaction times, facile catalyst recyclability, ambient temperature, and open-flask conditions made this catalytic system very practical. The iodide anions could form iodine bridges to construct stable dinuclear Cu(I) complexes with these ligands, which was the key to achieve high catalytic activities. While CuBr and CuCl were not suitable for this ligand system because of the improper size of Br and Cl atoms for the formation of the corresponding dinuclear Cu(I) complexes.
中文翻译:

吡啶基-三唑配体体系,用于高效CuI催化的叠氮化物-炔烃环加成反应
吡啶基-三唑配体系统(包括N 2 -2-吡啶基1,2,3-三唑和N 1 / N 2取代的2-(NH(1,2,3-三唑-4-基)吡啶被发现是CuI催化的叠氮化物-炔烃环加成(CuAAC)反应的优良配体。催化剂负载量低,反应时间短,催化剂易于回收利用,环境温度和开瓶条件使该催化系统非常实用。碘化物阴离子可形成碘桥,与这些配体一起构建稳定的双核Cu(I)配合物,这是实现高催化活性的关键。虽然CuBr和CuCl不适合该配体系统,因为Br和Cl原子的尺寸不合适,无法形成相应的双核Cu(I)配合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号