Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rethinking the Reaction Pathways of Chemical Reduction of Graphene Oxide
Carbon ( IF 10.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.carbon.2020.09.049 Jianqiang Guo , Boyang Mao , Jiongli Li , Xudong Wang , Xinzheng Yang
Carbon ( IF 10.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.carbon.2020.09.049 Jianqiang Guo , Boyang Mao , Jiongli Li , Xudong Wang , Xinzheng Yang
|
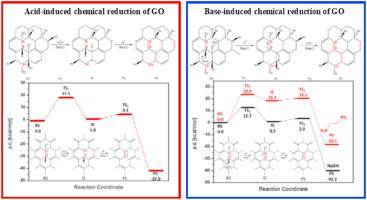
Abstract Although the methods of chemical reduction of graphene oxide have been intensively and well investigated, the reduction mechanism is still ambiguous to date. The reduction mechanisms proposed so far were limited to explain a specific reducing agent rather than a general accessible approach. Here, we suggest that the core issue of chemical reduction of graphene oxide is the reduction of hydroxyl groups. Fundamental reactions mechanisms - E1 and E1cB - are proposed and studied as a general applicable but conclusive reduction mechanisms. Density Functional Theory calculation was used to investigate both reaction pathways for the acid- and base-induced reductions. The calculation results show that both reaction pathways are favorable on kinetics and thermodynamics. By applying this reduction mechanism, we developed NaSH as a novel and efficient reducing agent to reduce GO at room temperature, which further confirms the feasibility of the proposed reaction pathways. This work is thus able to investigate possible reaction pathways for various chemical reduction methods and will help to mature novel reduction approaches.
中文翻译:

氧化石墨烯化学还原反应途径的再思考
摘要 尽管氧化石墨烯的化学还原方法已得到深入研究,但迄今为止还原机理仍不明确。迄今为止提出的还原机制仅限于解释特定的还原剂,而不是通用的可访问方法。在这里,我们建议氧化石墨烯化学还原的核心问题是羟基的还原。基本反应机制 - E1 和 E1cB - 作为普遍适用但决定性的还原机制被提出和研究。密度泛函理论计算用于研究酸和碱诱导的还原反应途径。计算结果表明,两种反应途径在动力学和热力学上都是有利的。通过应用这种减少机制,我们开发了 NaSH 作为一种新型有效的还原剂,可以在室温下还原 GO,这进一步证实了所提出的反应途径的可行性。因此,这项工作能够研究各种化学还原方法的可能反应途径,并将有助于成熟新的还原方法。
更新日期:2021-01-01
中文翻译:

氧化石墨烯化学还原反应途径的再思考
摘要 尽管氧化石墨烯的化学还原方法已得到深入研究,但迄今为止还原机理仍不明确。迄今为止提出的还原机制仅限于解释特定的还原剂,而不是通用的可访问方法。在这里,我们建议氧化石墨烯化学还原的核心问题是羟基的还原。基本反应机制 - E1 和 E1cB - 作为普遍适用但决定性的还原机制被提出和研究。密度泛函理论计算用于研究酸和碱诱导的还原反应途径。计算结果表明,两种反应途径在动力学和热力学上都是有利的。通过应用这种减少机制,我们开发了 NaSH 作为一种新型有效的还原剂,可以在室温下还原 GO,这进一步证实了所提出的反应途径的可行性。因此,这项工作能够研究各种化学还原方法的可能反应途径,并将有助于成熟新的还原方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号