Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.bioorg.2020.104297 Somayeh Zare , Hossein Mirkhani , Omidreza Firuzi , Niloofar Moheimanian , Mojtaba Asadollahi , Somayeh Pirhadi , Jima N. Chandran , Bernd Schneider , Amir Reza Jassbi
|
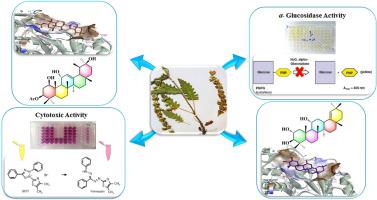
Two polyhydroxylated oleanane and seven ursane triterpenoids were isolated from aerial parts of Salvia grossheimii. The chemical structures of the undescribed triterpenoids (1–6) were characterized using 1 and 2 D NMR and ESI-MS spectral data as; 2α, 3β, 11α –trihydroxy-olean-12- ene (1), 2α, 3β, 11α-trihydroxy-olean-18-ene (2), 2α- acetoxy-urs-12-ene-3β, 11α, 20β-triol (3), 3-keto-urs-12-ene-1β, 11α, 20β -triol (4), 2α, 3β-diacetoxy-urs-12-ene-1β, 11α, 20β -triol (5), and 3β-acetoxy-urs-12-ene-1β, 11α, 20β –triol (6). All compounds were evaluated for the in vitro α-glucosidase inhibitory and cytotoxic activities against MCF-7 human cancer cell line. Compounds 1, 2, 4, and 6 showed in vitro α-glucosidase inhibitory activity with IC50 = 43.6–198.4 µM, which were more potent than the antidiabetic medicine, acarbose. The remaining compounds; 3, and 7–9 showed potent cytotoxic activity (IC50 = 6.2–31.9 µM) against the cancerous cell line, while the potent α-glucosidase inhibitors were inactive. Molecular docking analysis and kinetic studies were applied to investigate the structure activity relationships and mechanisms of the human and Saccharomyces cerevisiae α-glucosidase inhibitory of the purified compounds. Comparing the high cytotoxicity and α-glucosidase inhibitory of the oleanane and ursane type triterpenoids suggest them as potential lead compounds for further research in anticancer and antidiabetic research.
中文翻译:

鼠尾草的抗糖尿病和细胞毒性的多羟基戊烷和乌烷类三萜
从鼠尾草的地上部分分离出了两个多羟基的齐墩烷和七个熊醛三萜。的未描述的三萜类化合物(的化学结构1 - 6)使用图1和2 d NMR和ESI-MS光谱数据如进行了表征; 2 α,3 β,11 α三羟基齐墩果-12-烯(1),2 α,3 β,11 α三羟基齐墩果-18-烯(2),2 α -乙酰氧基URS -12-烯-3 β,11 α,20 β三醇(3),3-酮基- URS -12-烯-1-β,11 α,20 β三醇(4),2 α,3 β -diacetoxy-URS-12烯1 β,11 α,20 β三醇(5),和3 β乙酰氧基URS-12烯-1 β,11 α,20 β三醇(6)。评价所有化合物对MCF-7人癌细胞系的体外α-葡糖苷酶抑制作用和细胞毒活性。化合物1,2,4,和6显示出体外α-葡萄糖苷酶抑制活性,IC 50 = 43.6–198.4 µM,比抗糖尿病药物阿卡波糖更有效。其余化合物;3,和7 - 9显示了强的细胞毒活性(IC 50 针对癌性细胞系= 6.2-31.9μM),而有效的α葡糖苷酶抑制剂是无效的。应用分子对接分析和动力学研究来研究人类和酿酒酵母α-葡萄糖苷酶抑制纯化化合物的结构活性关系和机理。比较高细胞毒性和αβ-葡糖苷酶对齐墩果烷和乌苏烷型三萜的抑制作用表明它们是潜在的潜在抗癌和抗糖尿病研究的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号