Biomaterials ( IF 12.8 ) Pub Date : 2020-09-20 , DOI: 10.1016/j.biomaterials.2020.120409 Darren Svirskis , Georgina Procter , Manisha Sharma , Prabhat Bhusal , Anusha Dravid , Wiremu MacFater , Ahmed Barazanchi , Laura Bennet , Kaushik Chandramouli , Sree Sreebhavan , Priyanka Agarwal , Satya Amirapu , Jacqueline A. Hannam , Gavin P. Andrews , Andrew Hill , David S. Jones
|
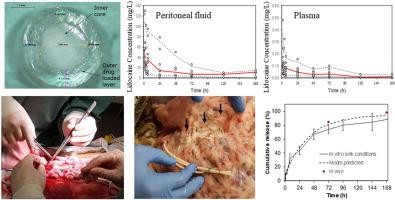
Appropriate management of post-operative pain is an ongoing challenge in surgical practice. At present, systemic opioid administration is routinely used for analgesia in the post-operative setting. However, due to significant adverse effects and potential for misuse, there is a perceived need for the development of alternative, opioid-sparing treatment modalities. Continuous infusion of local anesthetic into the peritoneum after major abdominal surgery reduces pain and opioid consumption, and enhances recovery from surgery. Here we describe, a non-opioid, poly(ethylene-co-vinyl-acetate) intraperitoneal implant for the sustained delivery of local anesthetic following major abdominal surgery. A radio-opaque core had the required mechanical strength to facilitate placement and removal procedures. This core was enclosed by an outer shell containing an evenly dispersed local anesthetic, lidocaine. Sustained release of lidocaine was observed in an ovine model over days and the movement modelled between peritoneal fluid and circulating plasma. While desirably high levels of lidocaine were achieved in the peritoneal space these were several orders of magnitude higher than blood levels, which remained well below toxic levels. A pharmacokinetic model is presented that incorporates in vitro release data to describe lidocaine concentrations in both peritoneal and plasma compartments, predicting similar release to that suggested by lidocaine concentrations remaining in the device after 3 and 7 days in situ. Histological analysis revealed similar inflammatory responses following implantation of the co-extruded implant and a commercially used silicone drain after three days. This non-opioid analgesic implant provides sustained release of lidocaine in an ovine model and is suitable for moving onto first in human trials.
中文翻译:

一种非阿片类镇痛植入物,用于持续术后利多卡因腹膜内递送,使用绵羊模型进行表征
术后疼痛的适当管理是外科手术中的持续挑战。目前,术后常规使用全身性阿片类药物进行镇痛。然而,由于严重的不良影响和滥用的可能性,人们认为需要开发替代性的,阿片类药物保留的治疗方式。大腹部手术后向腹膜连续输注局部麻醉药可减轻疼痛和阿片类药物的消耗,并增强手术恢复。在这里,我们描述了一种非阿片类药物,聚(乙烯-乙酸乙烯酯-乙烯-乙酸乙烯酯)腹膜内植入物,用于在重大腹部手术后持续输送局部麻醉药。不透射线的芯具有所需的机械强度,以方便放置和移除程序。该核被包含均匀分散的局部麻醉药利多卡因的外壳包围。在几天的绵羊模型中观察到利多卡因的持续释放,并且在腹膜液和循环血浆之间建立了运动模型。虽然在腹膜腔中达到了高水平的利多卡因水平,但它们比血液水平高出几个数量级,而血液水平仍远低于毒性水平。提出了一种药代动力学模型 仍远低于毒性水平。提出了一种药代动力学模型 仍远低于毒性水平。提出了一种药代动力学模型体外释放数据描述了腹膜腔和血浆腔中利多卡因的浓度,预测了与原位3天和7天后装置中剩余的利多卡因浓度所暗示的释放相似。组织学分析显示,植入共挤植入物和三天后市售的有机硅排出物后,炎症反应相似。这种非阿片类镇痛剂可在绵羊模型中持续释放利多卡因,适合在人体试验中首次应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号