Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.bbalip.2020.158814 Alexander D Dergunov 1 , Dmitry Y Litvinov 1 , Artem A Malkov 1 , Veronika B Baserova 1 , Elena V Nosova 2 , Liudmila V Dergunova 2
|
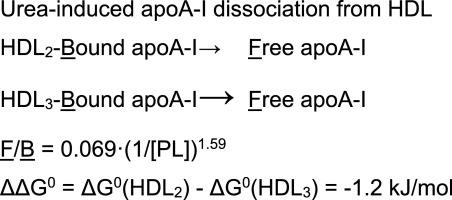
We studied the mechanism of HDL denaturation with concomitant apoA-I dissociation with HDL preparations from 48 patients with a wide range of plasma HDL-C and evaluated the contribution of lipid-free apoA-I into cholesterol efflux from macrophage, in particular, mediated by cholesterol transporter ABCA1. We prepared HDL by precipitation of apoB-containing lipoproteins by polyethylene glycol and used the chaotropic agent urea to denature HDL preparations. Apo-I dissociation from urea-treated HDL was assessed by the increase of preβ-band fraction with agarose gel electrophoresis followed by electro transfer and immunodetection and by the increase of ABCA1-mediated efflux of fluorescent analogue BODIPY-Cholesterol from RAW 264.7 macrophages. The HDL denaturation is governed by a single transition to fully dissociated apoA-I and the transition cooperativity decreases with increasing HDL-C. The apoA-I release depends on phospholipid concentration of HDL preparation and HDL compositional and structural heterogeneity and is well described by apolipoprotein partition between aqueous and lipid phases. Dissociated apoA-I determines the increase of ABCA1-mediated efflux of BODIPY-Cholesterol from RAW 264.7 macrophages to patient HDL. The increase in apoA-I dissociation is associated with the increase of ABCA1 gene transcript in peripheral blood mononuclear cells from patients. The low level of plasma HDL particles may be compensated by their increased potency for apoA-I release, thus suggesting apoA-I dissociation as a new HDL functional property.
中文翻译:

通过载脂蛋白AI分解研究尿素对人血浆高密度脂蛋白的变性。
我们研究了48名血浆HDL-C广泛的患者的HDL变性,伴随apoA-I解离和HDL制剂的伴随作用,并评估了无脂质apoA-I对巨噬细胞胆固醇外排的贡献,特别是通过胆固醇转运蛋白ABCA1。我们通过聚乙二醇沉淀含apoB的脂蛋白来制备HDL,并使用离液剂尿素使HDL制剂变性。尿素处理的HDL的Apo-I分解是通过琼脂糖凝胶电泳增加前β带的比例,随后进行电转移和免疫检测以及通过ABCA1介导的来自RAW 264.7巨噬细胞的荧光类似物BODIPY-胆固醇的外排的增加来评估的。HDL变性受单次转变为完全解离的apoA-I的控制,并且随着HDL-C的增加,转变的协同作用降低。apoA-I的释放取决于HDL制剂的磷脂浓度以及HDL组成和结构的异质性,并通过载脂蛋白在水相和脂质相之间的分配而得到很好的描述。分离的apoA-I决定了ABCA1介导的BODIPY-胆固醇从RAW 264.7巨噬细胞向患者HDL的流出增加。apoA-I解离的增加与 分离的apoA-I决定了ABCA1介导的BODIPY-胆固醇从RAW 264.7巨噬细胞向患者HDL的流出增加。apoA-I解离的增加与 离解的apoA-I决定了ABCA1介导的BODIPY-胆固醇从RAW 264.7巨噬细胞向患者HDL的流出增加。apoA-I解离的增加与患者外周血单个核细胞中的ABCA1基因转录本。血浆HDL颗粒的低含量可以通过其增加的apoA-I释放能力来补偿,因此表明apoA-I分解是一种新的HDL功能特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号